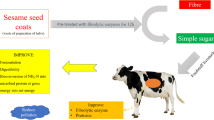
Abstract
Peanut hulls are abundant waste with high bioactive compounds, antioxidant activity, and cell wall polysaccharides but low nutritional value. The aim of this study was to valorize this agricultural waste into alternative ruminant feed with exogenous fibrolytic enzymes (EFE) produced by fermentation of mixture culture of Aspergillus strains (A. niger, A. tubingensis, A. oryzae, and A. sojae) and Neurospora intermedia. Peanut hulls were pretreated for 24 h with increasing EFE levels 0, 1, 2, and 4 mg/g dry matter. The results showed that the effectiveness of this additive depended on EFE level. The low EFE level did not affect their nutritional value. The moderate and high EFE levels converted part of their cell wall polysaccharide compound into non-fiber carbohydrates and solubilize their organic matter without altering their bioactive compounds and their antioxidant activity. Consequently, these two levels of EFE accelerate rumen fermentation process, reduce the time of onset of rumen fermentation, and improve cell wall polysaccharide digestibility, net energy lactation, and short-chain fatty acid production. However, only the high EFE level promoted the proliferation of rumen protozoa and the amount of fermentation and dry matter digestibility and reduced rumen ammonia nitrogen by conversion into microbial crude protein. In conclusion, this practical bioprocess with the highest EFE level can be used as an effective tool for bioconversion of these wastes into energy feeds with high bioactive compounds and antioxidant activity to substitute expensive ruminant feeds. This strategy can provide a new source of revenue for the peanut shelling and peanut oil industries and protect the environment from the pollution.
Graphical Abstract

Similar content being viewed by others
Data availability
The datasets and materials used during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ADF :
-
Acid detergent fiber
- ADL :
-
Acid detergent lignin
- B :
-
Asymptotic gas production
- C :
-
Constant gas production rate
- CP :
-
Crude protein
- DPPH :
-
2,2-Diphenyl-1-picrylhydrazyl
- EE :
-
Ether extract
- EFE :
-
Exogenous fibrolytic enzymes
- GP :
-
Net gas production
- GP24 :
-
Net gas production after 24 h of incubation
- Lag :
-
Time of onset of rumen fermentation
- NDF :
-
Neutral detergent fiber
- NFC :
-
Non-fiber carbohydrate
- SEM :
-
Standard error of means
- t:
-
Incubation time
References
García-Rodríguez J, Ranilla MJ, France J, Alaiz-Moretón H, Carro MD, López S (2019) Chemical composition, in vitro digestibility and rumen fermentation kinetics of agro-industrial by-products. Animals 9(11):861. https://doi.org/10.3390/ani9110861
Correddu F, Lunesu MF, Buffa G, Atzori AS, Nudda A, Battacone G, Pulina G (2020) Can agro-industrial by-products rich in polyphenols be advantageously used in the feeding and nutrition of dairy small ruminants? Animals 10:131. https://doi.org/10.3390/ani10010131
Oosting S, van der Lee J, Verdegem M, de Vries M, Vernooij A, Bonilla-Cedrez C, Kabir K (2022) Farmed animal production in tropical circular food systems. Food Secur 14:273–292. https://doi.org/10.1007/s12571-021-01205-4
Perea-Moreno MA, Manzano-Agugliaro F, Hernandez-Escobedo Q, Perea-Moreno AJ (2018) Peanut shell for energy: properties and its potential to respect the environment. Sustainability 10(9):3254. https://doi.org/10.3390/su10093254
Adhikari B, Dhungana SK, Ali MW, Adhikari A, Kim ID, Shin DH (2019) Antioxidant activities, polyphenol, flavonoid, and amino acid contents in peanut shell. J Saudi Soc Agric Sci 18(4):437–442. https://doi.org/10.1016/j.jssas.2018.02.004
Feng MM, Wang YF, Cai X, Zhang HC, Xu JX (2022) Changes in the physicochemical properties and in vitro protein digestibility of peanut hulls treated via mechanical activation. Food Sci Biotechnol 1-8.https://doi.org/10.1007/s10068-022-01084-1
Millam JJ, Bello SS, Abbaya HY, John PA (2020) Growth performance and serum biochemical profile in Yankasa rams fed alkali -treated groundnut shells. Fudma J Sci 3(4):55–59. https://doi.org/10.33003/fjs-2020-0403-251
Hill GM (2002) Peanut by-products fed to cattle. Vet Clin North Am Food Anim Pract 18(2):295–315. https://doi.org/10.1016/S0749-0720(02)00019-1
Yuan J, Wan X (2019) Multiple-factor associative effects of peanut shell combined with alfalfa and concentrate determined by in vitro gas production method. Czech J Anim Sci 64(8):352–360. https://doi.org/10.17221/94/2019-CJAS
Abo-Donia FM, Abdel-Azim SN, Elghandour MMY, Salem AZM, Buendía G, Soliman NAM (2014) Feed intake, nutrient digestibility and ruminal fermentation activities in sheep-fed peanut hulls treated with Trichoderma viride or urea. Trop Anim Health Prod 46(1):221–228. https://doi.org/10.1007/s11250-013-0479-z
Farooq S, Shah MA, Ganaie TA, Mir SA (2021) Exogenous enzymes. In: Gani A, Ashwar BA (eds) Food Biopolymers: Structural, Functional and Nutraceutical Properties. Springer Cham, Switzerland, pp 319–338
Abid K, Jabri J, Yaich H, Malek A, Rekhis J, Kamoun M (2022) In vitro study on the effects of exogenic fibrolytic enzymes produced from Trichoderma longibrachiatum on ruminal degradation of olive mill waste. Arch Anim Breed 65:79–88. https://doi.org/10.5194/aab-65-79-2022
Abid K, Jabri J, Yaich H, Malek A, Rekhis J, Kamoun M (2022) Improving the nutritional value and rumen fermentation characteristics of sesame seed coats through bioconversion approach using exogenous fibrolytic enzymes produced by Trichoderma longibrachiatum. Biomass Convers Biorefin 1-9.https://doi.org/10.1007/s13399-022-03402-3
Yang J, Guevara-Oquendo VH, Refat B, Yu P (2022) Effects of exogenous fibrolytic enzyme derived from Trichoderma reesei on rumen degradation characteristics and degradability of low-tannin whole plant faba bean silage in dairy cows. Dairy 3(2):303–313. https://doi.org/10.3390/dairy3020023
Zhang J, Wang C, Liu Q, Guo G, Huo W, Pei C, Jiang Q (2022) Influence of fibrolytic enzymes mixture on performance, nutrient digestion, rumen fermentation and microbiota in Holstein bulls. J Anim Feed Sci 31(1):46–54. https://doi.org/10.22358/jafs/147188/2022
Refat B, Christensen DA, McKinnon JJ, Yang W, Beattie AD, McAllister TA, Eun JS, Abdel-Rahman GA, Yu P (2018) Effect of fibrolytic enzymes on lactational performance, feeding behavior, and digestibility in high-producing dairy cows fed a barley silage–based diet. J Dairy Sci 101(9):7971–7979. https://doi.org/10.3168/jds.2017-14203
Abid K, Jabri J, Ammar H, Ben Said S, Yaich H, Malek A, Rekhis J, Lopez S, Kamoun M (2020) Effect of treating olive cake with fibrolytic enzymes on feed intake, digestibility and performance in growing lambs. Anim Feed Sci Tech 261:114405. https://doi.org/10.1016/j.anifeedsci.2020.114405
Sallam SM, Kholif AE, Amin KA, El-Din ANN, Attia MF, Matloup OH, Anele UY (2020) Effects of microbial feed additives on feed utilization and growth performance in growing Barki lambs fed diet based on peanut hay. Anim Biotechno 31(5):447–454. https://doi.org/10.1080/10495398.2019.1616554
Kholif AE, Hamdon HA, Gouda GA, Kassab AY, Morsy TA, Patra AK (2022) Feeding date-palm leaves ensiled with fibrolytic enzymes or multi-species probiotics to Farafra ewes: intake, digestibility, ruminal fermentation, blood chemistry, milk production and milk fatty acid profile. Animals 12(9):1107. https://doi.org/10.3390/ani12091107
Tirado-González DN, Tirado-Estrada G, Miranda-Romero LA, Ramírez-Valverde R, Medina-Cuéllar SE, Salem AZM (2021) Effects of addition of exogenous fibrolytic enzymes on digestibility and milk and meat production—a systematic review. Ann Anim Sci 21(4):1159–1192. https://doi.org/10.2478/aoas-2021-0001
Rosser C, Terry SA, Badhan A, McAllister TA, Beauchemin KA (2022) Current knowledge and future opportunities for ruminant enzymes. In: Bedford MR, Partridge GC, Hruby M, Walk CL (eds) Enzymes in farm animal nutrition. CABI Publishing, CAB International, London, UK. https://doi.org/10.1079/9781789241563.0000
Wang Y, Ramirez-Bribiesca JE, Yanke LJ, Tsang A, McAllister TA (2012) Effect of exogeous fibrolytic enzyme application on the microbial attachment and digestion of barley straw in vitro. Asian-Aust J Anim Sci 25(1):66–74. https://doi.org/10.5713/ajas.2011.11158
Yang J, Refat B, Guevara-Oquendo VH, Yu P (2022) Lactational performance, feeding behavior, ruminal fermentation and nutrient digestibility in dairy cows fed whole-plant faba bean silage-based diet with fibrolytic enzyme. Animal 16(9):100606. https://doi.org/10.1016/j.animal.2022.100606
Jabri J, Abid K, Yaich H, Malek A, Rekhis J, Kamoun M (2019) Effect of combining exogenous fibrolytics enzymes supplementation with alkali and acid pre-treatments on wheat straw hydrolysis and ruminal fermentation. Indian J Anim Sci 89(7):780–785
Baiely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23:257–270. https://doi.org/10.1016/0168-1656(92)90074-J
Wood TM, Bhat KM (1988) Methods for measuring cellulase activities. Methods Enzymol 160(87):112. https://doi.org/10.1016/0076-6879(88)60109-1
Association of Official Chemists Analytical Chemists (2000) Official methods of analysis, 17th edn. AOAC International, Gaithersburg, MD, USA
Van Soest PV, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74(10):3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Abid K, Jabri J, Beckers Y, Yaich H, Malek A, Rekhis J, Kamoun M (2019) Influence of adding fibrolytic enzymes on the ruminal fermentation of date palm by-products. Arch Anim Breed 62:1–8. https://doi.org/10.5194/aab-62-1-2019
National Research Council (2001) Nutrient requirements of dairy cattle, 7th edn. National Academies Press, Washington, USA. https://doi.org/10.17226/9825
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299:152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
Broadhurst RB, Jones WT (1978) Analysis of condensed tannins using acidified vanillin. J Sci Food Agric 29(9):788–794. https://doi.org/10.1002/jsfa.2740290908
Zhu H, Wang Y, Liu Y, Xia Y, Tang T (2010) Analysis of flavonoids in Portulaca oleracea L. by UV–vis spectrophotometry with comparative study on different extraction technologies. Food Anal Methods 3(2):90–97. https://doi.org/10.1007/s12161-009-9091-2
Xu BJ, Chang SKC (2007) A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J Food Sci 72(2):S159–S166. https://doi.org/10.1111/j.1750-3841.2006.00260.x
Theodorou MK, Williams BA, Dhanoa MS, McAllan AB, France JA (1994) Simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim Feed Sci Technol 48(3–4):185–197. https://doi.org/10.1016/0377-8401(94)90171-6
Menke KH, Steingass H (1988) Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev 28:7–55
Mauricio RM, Mould FL, Dhanoa MS, Owen E, Channa KS, Theodorou MK (1999) A semi-automated in vitro gas production technique for ruminant feedstuff evaluation. Anim Feed Sci Technol 79(4):321–330. https://doi.org/10.1016/S0377-8401(99)00033-4
France J, Dijkstra J, Dhanoa MS, Lopez S, Bannink A (2000) Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: derivation of models and other mathematical considerations. Br J Nutr 83:143–150. https://doi.org/10.1017/S0007114500000180
SAS Institute Inc (2011) SAS/STAT 9.3, user's guide. SAS Institute Inc, Cary, NC
Dehority BA (1993) Laboratory manual for classification and morphology of rumen ciliate protozoa. CRC Press. https://doi.org/10.1201/9781351073912
Broderick GA, Kang JH (1980) Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J Dairy Sci 63:64–75. https://doi.org/10.3168/jds.S0022-0302(80)82888-8
Getachew G, Blummel M, Makkar HPS, Becker K (1998) In vitro gas measuring techniques for assessment of nutritional quality of feeds: a review. Anim Feed Sci Tech 72:261–281. https://doi.org/10.1016/S0377-8401(97)00189-2
Blümmel M, Makkar HPS, Becker K (1997) In vitro gas production: a technique revisited. J Anim Physiol An N 77:24–34. https://doi.org/10.1111/j.1439-0396.1997.tb00734.x
Olagaray KE, Bradford BJ (2019) Plant flavonoids to improve productivity of ruminants–a review. Anim Feed Sci Technol 251:21–36. https://doi.org/10.1016/j.anifeedsci.2019.02.004
Gemeda BS, Hassen A (2015) Effect of tannin and species variation on in vitro digestibility, gas, and methane production of tropical browse plants. Asian-Australas J Anim Sci 28(2):188. https://doi.org/10.5713/ajas.14.0325
Yanza YR, Fitri A, Suwignyo B, Hidayatik N, Kumalasari NR, Irawan A, Jayanegara A (2021) The utilisation of tannin extract as a dietary additive in ruminant nutrition: a meta-analysis. Animals 11(11):3317. https://doi.org/10.3390/ani11113317
Akinfemi A, Adua MM, Adu OA (2012) Evaluation of nutritive values of tropical feed sources and by-products using in vitro gas production technique in ruminant animals. J Food Agric 24(4):348–353
Du W, Hou F, Tsunekawa A, Kobayashi N, Peng F, Ichinohe T (2020) Substitution of leguminous forage for oat hay improves nitrogen utilization efficiency of crossbred Simmental calves. J Anim Physiol Anim Nutr (Berl) 104(4):998–1009. https://doi.org/10.1111/jpn.13288
Van Soest PJ (1994) Nutritional ecology of the ruminant, 2nd edn. Cornell University Press, Ithaca, NY
Lynch JP, Jin L, Church JS, Baah J, Beauchemin KA (2013) Fibrolytic enzymes and a ferulic acid esterase-producing bacterial additive applied to alfalfa hay at baling: effects on fibre digestibility, chemical composition and conservation characteristics. Grass Forage Sci 70(1):85–93. https://doi.org/10.1111/gfs.12093
Kholif AE, Elghandour MMY, Rodríguez GB, Olafadehan OA, Salem AZM (2017) Anaerobic ensiling of raw agricultural waste with a fibrolytic enzyme cocktail as a cleaner and sustainable biological product. J Clean Prod 142(4):2649–2655. https://doi.org/10.1016/j.jclepro.2016.11.012
Kholif AE, Gouda GA, Morsy TA, Matloup OH, Fahmy M, Gomaa AS, Patra AK (2022) Dietary date palm leaves ensiled with fibrolytic enzymes decreased methane production, and improved feed degradability and fermentation kinetics in a ruminal in vitro system. Waste Biomass Valorization 13:3475–3488. https://doi.org/10.1007/s12649-022-01752-7
Li Z, Deng Q, Liu Y, Yan T, Li F, Cao Y, Yao J (2018) Dynamics of methanogenesis, ruminal fermentation and fiber digestibility in ruminants following elimination of protozoa: a meta-analysis. J Anim Sci Biotechnol 9(1):1–9. https://doi.org/10.1186/s40104-018-0305-6
Oba M, Allen MS (1999) Evaluation of the importance of the digestibility of neutral detergent fiber from forage: effects on dry matter intake and milk yield of dairy cows. J Dairy Sci 82(3):589–596. https://doi.org/10.3168/jds.S0022-0302(99)75271-9
Refat B, Christensen DA, Ismael A, Feng X, Rodríguez-Espinosa ME, Guevara-Oquendo VH, Yang J, AlZahal O, Yu P (2021) Evaluating the effects of fibrolytic enzymes on rumen fermentation, omasal nutrient flow, and production performance in dairy cows during early lactation. Can J Anim Sci 102(1):39–49. https://doi.org/10.1139/cjas-2020-0062
Jouany JP (2006) Optimizing rumen functions in the close-up transition period and early lactation to drive dry matter intake and energy balance in cows. Anim Reprod Sci 96(3–4):250–264. https://doi.org/10.1016/j.anireprosci.2006.08.005
Funding
This research was supported by the Laboratory of Animal Nutrition: Management of the Health and Quality of Animal Production [LR14AGR03] (Ministry of Higher Education and Scientific Research, Tunisia).
Author information
Authors and Affiliations
Contributions
Conceptualization, KA and MK; methodology, KA and MK; format analyses and investigation, KA, JJ, and HY; writing draft, KA; resource, AM, JR, and MK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The article does not contain any studies with human participants. It also does not perform experiments directly on animals. So, this experience does not need ethics statement.
Consent to participate
All the authors of this article are consented to participate.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abid, K., Jabri, J., Yaich, H. et al. Nutritional value assessments of peanut hulls and valorization with exogenous fibrolytic enzymes extracted from a mixture culture of Aspergillus strains and Neurospora intermedia. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03681-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03681-w