Abstract
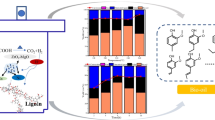
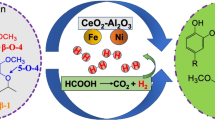
Lignin is the world’s greatest renewable aromatic biomass resource, with considerable potential for the development of high-value-added compounds. Herein, wet impregnation was employed to create Co-Mo/ATP-CZO bimetallic catalysts, which were then used to catalyze the depolymerization of lignin in ethanol and isopropanol. The findings of the characterization revealed that doping Mo metals resulted in the change of metallic oxide phase, which increased the oxygen vacancy (OV), improved metal-carrier interaction, and decreased acid center content. The results of the experiments demonstrated that catalytic hydrogenation with a 12Mo-20Co/ATP-CZO catalyst at 240 °C for 8 h produced the maximum yield of bio-oil (57.44 wt%, including 37.58 wt% of monomer). Under these conditions, the bio-oil had a higher heating value of 33.20 MJ/kg, which was significantly higher than the alkali lignin’s 19.62 MJ/kg. Catalyst stability tests also revealed good recyclability. This research will provide an efficient method for depolymerizing lignin.
Graphical abstract











Similar content being viewed by others
References
Iris K, Tsang DC (2017) Conversion of biomass to hydroxymethylfurfural: a review of catalytic systems and underlying mechanisms. Biores Technol 238:716–732. https://doi.org/10.1016/j.biortech.2017.04.026
Pandey MP, Kim CS (2011) Lignin depolymerization and conversion: a review of thermochemical methods. Chem Eng Technol 34:29–41. https://doi.org/10.1002/ceat.201000270
Liu X, Jiang Z, Feng S, Zhang H, Li J, Hu C (2019) Catalytic depolymerization of organosolv lignin to phenolic monomers and low molecular weight oligomers. Fuel 244:247–257. https://doi.org/10.1016/j.fuel.2019.01.117
Sun RC (2020) Lignin source and structural characterization. Chemsuschem 13:4385–4393. https://doi.org/10.1002/cssc.202001699
Naik SN, Goud VV, Rout PK, Dalai AK (2010) Production of first and second generation biofuels: a comprehensive review. Renew Sustain Energy Rev 14:578–597. https://doi.org/10.1016/j.fuel.2020.118048
Gosselink RJ, Teunissen W, Van Dam JE, De Jong E, Gellerstedt G, Scott EL, Sanders JP (2012) Lignin depolymerisation in supercritical carbon dioxide/acetone/water fluid for the production of aromatic chemicals. Biores Technol 106:173–177. https://doi.org/10.1016/j.biortech.2011.11.121
Sergeev AG, Webb JD, Hartwig JF (2012) A heterogeneous nickel catalyst for the hydrogenolysis of aryl ethers without arene hydrogenation. J Am Chem Soc 134:20226–20229. https://doi.org/10.1021/ja3085912
Hidajat MJ, Riaz A, Park J, Insyani R, Verma D, Kim J (2017) Depolymerization of concentrated sulfuric acid hydrolysis lignin to high-yield aromatic monomers in basic sub-and supercritical fluids. Chem Eng J 317:9–19. https://doi.org/10.1016/j.cej.2017.02.045
Guan W, Chen X, Hu H, Tsang C-W, Zhang J, Lin CSK, Liang C (2020) Catalytic hydrogenolysis of lignin β-O-4 aryl ether compound and lignin to aromatics over Rh/Nb2O5 under low H2 pressure. Fuel Process Technol 203:106392. https://doi.org/10.1016/j.fuproc.2020.106392
Song Q, Wang F, Cai J, Wang Y, Zhang J, Yu W, Xu J (2013) Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ Sci 6:994–1007. https://doi.org/10.1039/C2EE23741E
Rautiainen S, Di Francesco D, Katea SN, Westin G, Tungasmita DN, Samec JS (2019) Lignin valorization by cobalt-catalyzed fractionation of lignocellulose to yield monophenolic Compounds. Chemsuschem 12:404–408. https://doi.org/10.1002/cssc.201802497
Huang X, Korányi TI, Boot MD, Hensen EJ (2014) Catalytic depolymerization of lignin in supercritical ethanol. Chemsuschem 7:2276–2288. https://doi.org/10.1002/cssc.201402094
Chen M, Tang Z, Wang Y, Shi J, Li C, Yang Z, Wang J (2022) Catalytic depolymerization of Kraft lignin to liquid fuels and guaiacol over phosphorus modified Mo/Sepiolite catalyst. Chem Eng J 427:131761. https://doi.org/10.1016/j.cej.2021.131761
Haden WL, Schwint IA (1967) Attapulgite: its properties and applications. Ind Eng Chem 59:58–69
Guo H, Zhang H, Peng F, Yang H, Xiong L, Wang C, Huang C, Chen X, Ma L (2015) Effects of Cu/Fe ratio on structure and performance of attapulgite supported CuFeCo-based catalyst for mixed alcohols synthesis from syngas. Appl Catal A 503:51–61. https://doi.org/10.1016/j.clay.2015.03.009
Cao J-L, Shao G-S, Wang Y, Liu Y, Yuan Z-Y (2008) CuO catalysts supported on attapulgite clay for low-temperature CO oxidation. Catal Commun 9:2555–2559. https://doi.org/10.1016/j.catcom.2008.07.016
Guo H, Zhang H, Peng F, Yang H, Xiong L, Huang C, Wang C, Chen X, Ma L (2015) Mixed alcohols synthesis from syngas over activated palygorskite supported Cu–Fe–Co based catalysts. Appl Clay Sci 111:83–89. https://doi.org/10.1016/j.apcata.2015.07.008
Liang C, Gao Z, Lian H, Li X, Zhang S, Liu Q, Dong D, Hu X (2020) Impacts of metal loading in Ni/attapulgite on distribution of the alkalinity sites and reaction intermediates in CO2 methanation reaction. Int J Hydrogen Energy 45:16153–16160. https://doi.org/10.1016/j.ijhydene.2020.04.070
Yan Z, Liu Q, Liang L, Ouyang J (2021) Surface hydroxyls mediated CO2 methanation at ambient pressure over attapulgite-loaded Ni-TiO2 composite catalysts with high activity and reuse ability. J CO2 Utilization 47:101489. https://doi.org/10.1016/j.jcou.2021.101489
Tian H, Guo Q, Xu D (2010) Hydrogen generation from catalytic hydrolysis of alkaline sodium borohydride solution using attapulgite clay-supported Co-B catalyst. J Power Sources 195:2136–2142. https://doi.org/10.1016/j.jpowsour.2009.10.006
Wang Y, Chen M, Li X, Yang Z, Liang T, Zhou Z, Cao Y (2018) Hydrogen production via steam reforming of ethylene glycol over Attapulgite supported nickel catalysts. Int J Hydrogen Energy 43:20438–20450. https://doi.org/10.1016/j.ijhydene.2018.09.109
Chen T, Liu H, Shi P, Chen D, Song L, He H, Frost RL (2013) CO2 reforming of toluene as model compound of biomass tar on Ni/Palygorskite. Fuel 107:699–705. https://doi.org/10.1016/j.fuel.2012.12.036
Liu H, Chen T, Chang D, Chen D, Kong D, Zou X, Frost RL (2012) Effect of preparation method of palygorskite-supported Fe and Ni catalysts on catalytic cracking of biomass tar. Chem Eng J 188:108–112. https://doi.org/10.1016/j.cej.2012.01.109
Guo H, Zhang H, Zhang L, Wang C, Peng F, Huang Q, Xiong L, Huang C, Ouyang X, Chen X (2018) Selective hydrogenation of furfural to furfuryl alcohol over acid-activated attapulgite-supported NiCoB amorphous alloy catalyst. Ind Eng Chem Res 57:498–511. https://doi.org/10.1021/acs.iecr.7b03699
Guo H, Zhang H, Chen X, Zhang L, Huang C, Li H, Peng F, Huang Q, Xiong L, Ouyang X (2018) Catalytic upgrading of biopolyols derived from liquefaction of wheat straw over a high-performance and stable supported amorphous alloy catalyst. Energy Convers Manage 156:130–139. https://doi.org/10.1016/j.enconman.2017.11.006
Dar BA, Khalid S, Wani TA, Mir MA, Farooqui M (2015) Ceria-based mixed oxide supported CuO: an efficient heterogeneous catalyst for conversion of cellulose to sorbitol. Green and Sustainable Chemistry 5:15. https://doi.org/10.4236/gsc.2015.51003
Grams J, Niewiadomski M, Ryczkowski R, Ruppert AM, Kwapiński W (2016) Activity and characterization of Ni catalyst supported on CeO2–ZrO2 for thermo-chemical conversion of cellulose. Int J Hydrog Energy 41:8679–8687. https://doi.org/10.1016/j.ijhydene.2015.11.140
Schimming SM, LaMont OD, König M, Rogers AK, D’Amico AD, Yung MM, Sievers C (2015) Hydrodeoxygenation of guaiacol over ceria–zirconia catalysts. Chemsuschem 8:2073–2083. https://doi.org/10.1002/cssc.201500317
Yoshikawa T, Shinohara S, Yagi T, Ryumon N, Nakasaka Y, Tago T, Masuda T (2014) Production of phenols from lignin-derived slurry liquid using iron oxide catalyst. Appl Catal B 146:289–297. https://doi.org/10.1016/j.apcatb.2013.03.010
Prieto P, Ferreira A, Haddad P, Zanchet D, Bueno J (2010) Designing Pt nanoparticles supported on CeO2–Al2O3: synthesis, characterization and catalytic properties in the steam reforming and partial oxidation of methane. J Catal 276:351–359. https://doi.org/10.1016/j.jcat.2010.09.025
Amairia C, Fessi S, Ghorbel A, Rîves A (2010) Methane oxidation behaviour over sol–gel derived Pd/Al2O3-ZrO2 materials: influence of the zirconium precursor. J Mol Catal A: Chem 332:25–31. https://doi.org/10.1016/j.molcata.2010.08.013
Chen Q-Y, Li N, Luo M-F, Lu J-Q (2012) Catalytic oxidation of dichloromethane over Pt/CeO2–Al2O3 catalysts. Appl Catal B 127:159–166. https://doi.org/10.1016/j.apcatb.2012.08.020
Barbelli ML, Pompeo F, Santori GF, Nichio NN (2013) Pt catalyst supported on α-Al2O3 modified with CeO2 and ZrO2 for aqueous-phase-reforming of glycerol. Catal Today 213:58–64. https://doi.org/10.1016/j.cattod.2013.02.023
Guo H, Li Q, Zhang H, Peng F, Xiong L, Yao S, Huang C, Chen X (2019) CO2 hydrogenation over acid-activated attapulgite/Ce0.75Zr0.25O2 nanocomposite supported Cu-ZnO based catalysts. Mol Catal 476:110499. https://doi.org/10.1016/j.mcat.2019.110499
Chen J, Wang D, Lu X, Guo H, Xiu P, Qin Y, Xu C and Gu X (2022) Effect of cobalt (II) on acid-modified attapulgite-supported catalysts on the depolymerization of alkali lignin. Ind Eng Chem Res https://doi.org/10.1021/acs.iecr.1c04695
Wang J, Li W, Wang H, Ma Q, Li S, H-m C, Jameel H (2017) Liquefaction of kraft lignin by hydrocracking with simultaneous use of a novel dual acid-base catalyst and a hydrogenation catalyst. Biores Technol 243:100–106. https://doi.org/10.1016/j.biortech.2017.06.024
Li W, Dou X, Zhu C, Wang J, Chang H-m, Jameel H, Li X (2018) Production of liquefied fuel from depolymerization of kraft lignin over a novel modified nickel/H-beta catalyst. Biores Technol 269:346–354. https://doi.org/10.1016/j.biortech.2018.08.125
Zhu C, Dou X, Li W, Liu X, Li Q, Ma J, Liu Q, Ma L (2019) Efficient depolymerization of Kraft lignin to liquid fuels over an amorphous titanium-zirconium mixed oxide supported partially reduced nickel-cobalt catalyst. Biores Technol 284:293–301. https://doi.org/10.1016/j.biortech.2019.03.126
Cui K, Yang L, Ma Z, Yan F, Wu K, Sang Y, Chen H, Li Y (2017) Selective conversion of guaiacol to substituted alkylphenols in supercritical ethanol over MoO3. Appl Catal B 219:592–602. https://doi.org/10.1016/j.apcatb.2017.08.009
Prasomsri T, Shetty M, Murugappan K, Román-Leshkov Y (2014) Insights into the catalytic activity and surface modification of MoO3 during the hydrodeoxygenation of lignin-derived model compounds into aromatic hydrocarbons under low hydrogen pressures. Energy Environ Sci 7:2660–2669. https://doi.org/10.1039/C4EE00890A
Ressler T, Jentoft RE, Wienold J, Günter MM, Timpe O (2000) In situ XAS and XRD studies on the formation of Mo suboxides during reduction of MoO3. J Phys Chem B 104:6360–6370. https://doi.org/10.1021/jp000690t
Li X, Ni C, Yao C, Chen Z (2012) Development of attapulgite/Ce1−xZrxO2 nanocomposite as catalyst for the degradation of methylene blue. Appl Catal B 117:118–124. https://doi.org/10.1016/j.apcatb.2012.01.008
St GC, Stoyanova M, Georgieva M, Mehandjiev D (1999) Preparation and characterization of a higher cobalt oxide. Mater Chem Phys 60:39–43. https://doi.org/10.1016/S0254-0584(99)00053-X
Chithambararaj A, Rajeswari Yogamalar N, Bose AC (2016) Hydrothermally synthesized h-MoO3 and α-MoO3 nanocrystals: new findings on crystal-structure-dependent charge transport. Cryst Growth Des 16:1984–1995. https://doi.org/10.1021/acs.cgd.5b01571
Ding Q, Huang H, Duan J, Gong J, Yang S, Zhao X, Du Y (2006) Molybdenum trioxide nanostructures prepared by thermal oxidization of molybdenum. J Cryst Growth 294:304–308. https://doi.org/10.1016/j.jcrysgro.2006.07.004
Gao L, Li C, Zhang J, Du X, Li S, Zeng J, Yi Y, Zeng G (2018) Simultaneous removal of NO and Hg0 from simulated flue gas over CoOx-CeO2 loaded biomass activated carbon derived from maize straw at low temperatures. Chem Eng J 342:339–349. https://doi.org/10.1016/j.cej.2018.02.100
Wang C, Zhang C, Hua W, Guo Y, Lu G, Gil S, Giroir-Fendler A (2017) Catalytic oxidation of vinyl chloride emissions over Co-Ce composite oxide catalysts. Chem Eng J 315:392–402. https://doi.org/10.1016/j.cej.2018.02.100
Zhang X, Tang J, Zhang Q, Liu Q, Li Y, Chen L, Wang C, Ma L (2019) Hydrodeoxygenation of lignin-derived phenolic compounds into aromatic hydrocarbons under low hydrogen pressure using molybdenum oxide as catalyst. Catal Today 319:41–47. https://doi.org/10.1016/j.cattod.2018.03.068
Lai W, Pang L, Zheng J, Li J, Wu Z, Yi X, Fang W, Jia L (2013) Efficient one pot synthesis of mesoporous NiMo–Al2O3 catalysts for dibenzothiophene hydrodesulfurization. Fuel Process Technol 110:8–16. https://doi.org/10.1016/j.fuproc.2013.01.006
Chen M, Ma X, Ma R, Wen Z, Yan F, Cui K, Chen H, Li Y (2017) Ethanolysis of kraft lignin over a reduction-modified MoO3 catalyst. Ind Eng Chem Res 56:14025–14033. https://doi.org/10.1021/acs.iecr.7b03585
Chen N, Gong S, Qian EW (2015) Effect of reduction temperature of NiMoO3-x/SAPO-11 on its catalytic activity in hydrodeoxygenation of methyl laurate. Appl Catal B 174:253–263. https://doi.org/10.1016/j.apcatb.2015.03.011
González J, Wang JA, Chen L, Manríquez M, Salmones J, Limas R, Arellano U (2018) Quantitative determination of oxygen defects, surface lewis acidity, and catalytic properties of mesoporous MoO3/SBA-15 catalysts. J Solid State Chem 263:100–114. https://doi.org/10.1016/j.jssc.2018.04.005
Choi J-G, Thompson L (1996) XPS study of as-prepared and reduced molybdenum oxides. Appl Surf Sci 93:143–149. https://doi.org/10.1016/0169-4332(95)00317-7
Oregui-Bengoechea M, Gandarias I, Miletić N, Simonsen SF, Kronstad A, Arias PL, Barth T (2017) Thermocatalytic conversion of lignin in an ethanol/formic acid medium with NiMo catalysts: Role of the metal and acid sites. Appl Catal B 217:353–364. https://doi.org/10.1016/j.apcatb.2017.06.004
Huang X, Atay C, Korányi TI, Boot MD, Hensen EJ (2015) Role of Cu–Mg–Al mixed oxide catalysts in lignin depolymerization in supercritical ethanol. ACS Catal 5:7359–7370. https://doi.org/10.1021/acscatal.5b02230
Raikwar D, Munagala M, Majumdar S, Shee D (2019) Hydrodeoxygenation of guaiacol over Mo, W and Ta modified supported nickel catalysts. Catal Today 325:117–130. https://doi.org/10.1016/j.cattod.2018.09.039
Zhang C, Li H, Lu J, Zhang X, MacArthur KE, Heggen M, Wang F (2017) Promoting lignin depolymerization and restraining the condensation via an oxidation− hydrogenation strategy. ACS Catal 7:3419–3429. https://doi.org/10.1021/acscatal.7b00148
Shu R, Zhang Q, Ma L, Xu Y, Chen P, Wang C, Wang T (2016) Insight into the solvent, temperature and time effects on the hydrogenolysis of hydrolyzed lignin. Biores Technol 221:568–575. https://doi.org/10.1016/j.biortech.2016.09.043
Yuan Z, Cheng S, Leitch M, Xu CC (2010) Hydrolytic degradation of alkaline lignin in hot-compressed water and ethanol. Biores Technol 101:9308–9313. https://doi.org/10.1016/j.biortech.2010.06.140
Funding
This research was financially supported by National Natural Science Foundation of China (No. 21774059).
Author information
Authors and Affiliations
Contributions
J. C. and P. X. performed experiments. J. C. and X. G. conceived research, analyzed data, and wrote the manuscript with help from all the others.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Co-Mo bimetallic catalyst exhibited excellent activity on lignin depolymerization.
• The addition of Mo promoted the production of fuel and phenol monomers from lignin.
• Co-Mo/ATP-CZO catalyst shown higher selectivity compared with Co/ATP-CZO.
• Maximally 57.44 wt% of monomeric and dimeric degradation products was obtained.
• The HHV of liquid product was increased from 28.35 to 33.20 MJ/kg.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, J., Xiu, P. & Gu, X. Hydrogenolysis of alkali lignin via a non-precious Co-Mo bimetallic catalyst supported on attapulgite-Ce0.75Zr0.25O2. Biomass Conv. Bioref. 14, 11389–11401 (2024). https://doi.org/10.1007/s13399-022-03166-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03166-w