Abstract
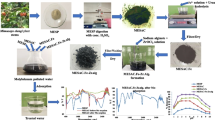
Nanoparticles of mixed n(Zr-Ce-Sm) oxide of crystalline size 11.7 nm are successfully synthesized by new green methods adopting Aloe vera gel as capping agent and evoking the principles of classical methods of homogenous precipitations. An active carbon is derived from stems of Artocarpus heterophyllus plant. The Artocarpus heterophyllus active carbon (AHAC), active carbon loaded with nanoparticles ‘AHAC + n(Zr-Ce-Sm) oxide’ and iron alginate beads doped with their admixture, ‘AHAC + n(Zr-Ce-Sm) oxide-Fe.Ali’, are investigated for their effectiveness for the simultaneous removal of lead and cadmium ions from water. Different extraction conditions are optimized for the maximum efficiency of the three sorbents by batch methods. The sorbents have shown good sorption nature for both the toxic ions at the pHs: 6.0 for active carbon, 6.5 for admixture and 7.0 for beads respectively at different equilibration times and dosages. The maximum adsorption capacities for Pb2+/Cd2+ ions are as follows: 15.0/20.0, 28.0/32.6, 34.0/44.3 mg/g for AHAC, admixture and beads respectively. The sorbents are characterized for various physicochemical parameters and structural aspects using XRD, FTIR, EDX and FESEM. The effects of co-ions on the simultaneous extraction are investigated. The mechanism of the adsorption process is analysed based on thermodynamic data and applicability of isotherms and kinetic models. Based on these, the mechanism of adsorption process involves the formation of a surface complex and/or ion-exchange between Pb2+/Cd2+ with the surface functional groups of adsorbents. The same is confirmed by IR investigations. The spent adsorbents can be regenerated and reused. The adsorbents developed are successfully used to treat the industrial waste water samples. The merit of this investigation is that the effective sorbents with high adsorption capacities are developed for the simultaneous remove of the highly toxic lead and cadmium ions from industrial wastewater.
Graphical abstract












Similar content being viewed by others
References
Abdulrazak S, Hussaini K, Sani HM (2017) Evaluation of removal efficiency of heavy metals by low-cost activated carbon prepared from African palm fruit. Appl Water Sci 7(6):3151–3155. https://doi.org/10.1007/s13201-016-0460
Adamson AW, Gast AP (1997) Physical Chemistry of Surfaces, 6th Edition, Wiley, New York. https://doi.org/10.1126/science.160.3824.179.
Adeyanju CA, Ogunniyi S, Selvasembian R, Oniye MM, Ajala OJ, Adeniyi AG, Igwegbe CA, Ighalo JO (2022) Recent advances on the aqueous phase adsorption of carbamazepine. Chem Bio Eng Reviews. https://doi.org/10.1002/cben.202100042
Aharoni C, Ungarish M (1977) Kinetics of activated chemisorption. Part 2. Theoretical models. J Chem Soc, Faraday Trans 1: Phys Chemi Condensed Phases 73:456–464. https://doi.org/10.1039/F19777300456
Alslaibi TM, Abustan I, Ahmad MA, Abu Foul A (2015) Comparative studies on the olive stone activated carbon adsorption of Zn2+, Ni2+, and Cd2+ from synthetic wastewater. Desalination Water Treat 3;54(1):166–77. https://doi.org/10.1080/19443994.2013.876672
Amarsingh Bhabu K, Theerthagiri J, Madhavan J, Balu T, Muralidharan G, Rajasekaran TR (2015) Cubic fluorite phase of samarium doped cerium oxide (CeO2)0.96Sm0.04 for solid oxide fuel cell electrolyte. J Mater Sci Mater Electron 27:1566–1573. https://doi.org/10.1007/s10854-015-3925-z
Amin MT, Alazba AA, Shafiq M (2021) Comparative removal of lead and nickel ions onto nanofibrous sheet of activated polyacrylonitrile in batch adsorption and application of conventional kinetic and isotherm models. Membranes 11(1):10. https://doi.org/10.3390/membranes11010010
Amina R, Abdelkader K, Mehdi A, Zoubida T, Djamila I, Safia T, Andre D (2020) Lead and cadmium removal by adsorption process using hydroxyapatite porous materials. Water Practice Technol 15(1). https://doi.org/10.2166/wpt.2020.003
APHA (1998) Standard methods for the examination of water and waste water. APHA, Washington, DC
ASTM D4607–94 (2006) Standard test method for determination of iodine number of activated carbons. In American Society for Testing and Materials, pp 1–5
Badessa TS, Wakuma E, Yimer AM (2020) Bio-sorption for effective removal of chromium(VI) from wastewater using Moringa stenopetala seed powder (MSSP) and banana peel powder (BPP). BMC Chemistry 14(1):1–2. https://doi.org/10.1186/s13065-020-00724-z
Biftu WK, Suneetha M, Ravindhranath K (2020) De-fluoridation of polluted water using aluminium alginate beads doped with green synthesized ‘nano SiO2 + nano CeO2-ZrO2’, as an effective adsorbent. Chemistry Select 5(47):15061–15074. https://doi.org/10.1002/slct.202003744
Biftu WK, Suneetha M, Ravindhranath K (2021) Sequential adsorptive removal of phosphate, nitrate and chromate from polluted water using active carbon derived from stems of Carissa carandas plant. Water Practice Technol 16(1):117–134. https://doi.org/10.2166/wpt.2020.102 (IWA Publishing)
Biftu WK, Suneetha M, Ravindhranath K (2021b) Zirconium-alginate beads doped with H2SO4-activated carbon derived from leaves of Magnoliaceae plant as an effective adsorbent for the removal of chromate. Biomass Convers Biorefin. 1–16. https://doi.org/10.1007/s13399-021-01568-w
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60(2):309–19. https://doi.org/10.1021/ja01269a023
Bureau of Indian Standards (1989) Granular: methods of sampling and tests, New Delhi, India ISI, p. 877
Chien SH, Clayton WR (1980) Application of the Elovitch equation to the kinetics of phosphorus release and sorption in soils. Soil Sci Soc Am J 44:265–268
Coates, J. (2000) ‘Interpretation of infrared spectra, a practical approach’, in Meyer, R.A. (Ed.): Encyclopedia of Analytical Chemistry, pp.490–517, Wiley, Chicester.
Devi PV, Suneetha M, Ravindhranath K (2019) Effective activated carbon as adsorbent for the removal of copper (II) ions from wastewater. Asian J Chem 31(10):2233–2239
Dorofeev GA, Streletskii AN, Povstugar IV, Protasov AV, Elsukov EP (2012) Determination of nanoparticle sizes by X-ray diffraction. Colloid J 74(6):675–685. https://doi.org/10.1134/S1061933X12060051
Dubinin MM (1947) The equation of the characteristic curve of activated charcoal. InDokl Akad Nauk SSSR 55:327–329
El-Hendawy AN, Samra SE, Girgis BS (2001) Adsorption characteristics of activated carbons obtained from corncobs. Colloids Surf A 180(3):209–221. https://doi.org/10.1016/S0927-7757(00)00682-8
Farooq A, Elsayed F, Naveed A (2014) Removal of lead from wastewater by adsorption using Saudi Arabian clay. Int J Chem Environ Eng 5(2):65–68
Francisco JA, Lorena A, Irene G, Felix AL (2018) Removal of Pb2+ in Wastewater via adsorption onto an activated carbon produced from winemaking waste. Metals 8:697. https://doi.org/10.3390/met8090697
Freundlich HM (1906) Over the adsorption in solution. J Phys Chem 57(385471):1100–1107
Gao W, Wang Y, Basavanagoud B, Jamil MK (2017) Characteristics studies of molecular structures in drugs. Saudi Pharm J 25(4):580–6. https://doi.org/10.1016/j.jsps.2017.04.027
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore-and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind Eng Chem Fundam 5(2):212–23. https://doi.org/10.1021/i160018a011
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465. https://doi.org/10.1007/s11356-019-05050
Igwegbe CA, Onukwuli OD, Ighalo JO, Okoye PU (2020) Adsorption of cationic dyes on Dacryodes edulis seeds activated carbon modified using phosphoric acid and sodium chloride. Environ Processes. https://doi.org/10.1007/s40710-020-00467-y
Igwegbe CA, Ighalo JO, Ghosh S, Ahmadi S, Ugonabo VI (2021) Pistachio (Pistacia vera) waste as adsorbent for wastewater treatment: a review. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-01739-9
Jafari Kang A, Baghdadi M, Pardakhti A (2016) Removal of cadmium and lead from aqueous solutions by magnetic acid-treated activated carbon nanocomposite. Desalin Water Treat 57(40):18782–98. https://doi.org/10.1080/19443994.2015.1095123
Jellali S, Azzaz AA, Jeguirim M, Hamdi H, Mlayah A (2021) Use of lignite as a low-cost material for cadmium and copper removal from aqueous solutions: assessment of adsorption characteristics and exploration of involved mechanisms. Water 13:164. https://doi.org/10.3390/w13020164
Kishore Babu D, Ravindhranath K, Suneetha M (2021) Simple effective new bio-adsorbents for the removal of highly toxic nitrite ions from waste water. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-01677-6
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. Kungliga Svenska, Vetenskapsakademiens. Handlingar, 24(4), 1-39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(9):1361–403. https://doi.org/10.1021/ja02242a004
Lata S, Singh PK, Samadder SR (2015) Regeneration of adsorbents and recovery of heavy metals: a review. Int J Environ Sci Technol 12:1461–1478. https://doi.org/10.1007/s13762-014-0714-9
Li B, Zhou F, Huang K, Wang Y, Mei S, Zhou Y, Jing T (2016) Highly efficient removal of lead and cadmium during wastewater irrigation using a polyethylenimine-grafted gelatin sponge. Sci Rep 6:33573. https://doi.org/10.1038/srep33573
Maryam A, Davood H, Somayyeh H, Vahideh K (2021) The nano-magnetite-loaded 2-mercaptobenzoxazole as an adsorbent for the selective removal of the Pb2+, Ni2+ and Cd2+ ions from aqueous solutions. Korean J Chem Eng 38(7):1510–1521. https://doi.org/10.1007/s11814-021-0792-6
Mekonnen DT, Alemayehu E, Lennartz B (2021) Adsorptive removal of phosphate from aqueous solutions using low-cost volcanic rocks: kinetics and equilibrium approaches. Materials 14:1312. https://doi.org/10.3390/ma14051312
Momina M, Mohammad S, Suzylawati I (2018) Regeneration performance of clay-based adsorbents for the removal of industrial dyes: a review. RSC Adv 8:24571–24587. https://doi.org/10.1039/c8ra04290j
Mukherjee S, Kumari D, Joshi M, An AK, Kumar M (2020) Low-cost bio-based sustainable removal of lead and cadmium using a polyphenolic bioactive Indian curry leaf (Murrayakoengii) powder. Int J Hyg Environ Health 226:113471. https://doi.org/10.1016/j.ijheh.2020.113471
Mukul CAC, Abdellaoui Y, Abatal M, Vargas J, Santiago AA, Barron-Zambrano JA (2019) Eco-efficient biosorbent based on Leucaena leucocephala residues for the simultaneous removal of Pb(II) and Cd(II) ions from water system: sorption and mechanism. Bioinorg Chem Appl. 1–13. https://doi.org/10.1155/2019/2814047
Naeema HY (2014) Removal of lead (II) from waste water by adsorption. Int J Curr Microbiol App Sci 3(4):207–228
Namasivayam C, Kadirvelu K (1997) Activated carbons prepared from coir pith by physical and chemical activation methods. Bioresour Technol 62(3):123–7. https://doi.org/10.1016/S0960-8524(97)00074-6
Onyango MS, Kojima Y, Aoyi O, Bernardo EC, Matsuda H (2004) Adsorption equilibrium modeling and solution chemistry dependence of fluoride removal from water by trivalent-cation-exchanged zeolite F-9. J Colloid Interface Sci 279(2):341–50. https://doi.org/10.1016/j.jcis.2004.06.038
Pentari D, Vamvouka D (2019) Cadmium removal from aqueous solutions using nano-iron doped lignite. IOP Conf Ser Earth Environ Sci 221:012135
Reddy JB, Sneha Latha P, Biftu WK, Suneetha M, Ravindhranath K (2021) Effective removal of Cu2+ ions from polluted water using new bio-adsorbents. Water Practice Technol 16(2):566–581. https://doi.org/10.2166/wpt.2021.019 (IWA Publishing)
Renu, MA, Singh K (2016) Heavy metal removal from wastewater using various adsorbents: A review. J Water Reuse Desalination 7(4):387–419. https://doi.org/10.2166/WRD.2016.104
Singanan M (2011) Removal of lead (II) and cadmium (II) ions from wastewater using activated biocarbon. ScienceAsia 37:115–119. https://doi.org/10.2306/scienceasia1513-1874.2011.37.115
Sneha Latha P, Biftu WK, Suneetha M, Ravindhranath K (2022) Adsorptive removal of toxic chromate and phosphate ions from polluted water using green synthesized nano metal (Mn-Al-Fe) oxide. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-02293-0
Sneha Latha P, Biftu WK, Suneetha M, Ravindhranath K (2021a) Simultaneous removal of Lead and cadmium ions from simulant and industrial waste water: using Calophylluminophyllum plant materials as sorbents. Int J Phytoremediation 1–15. https://doi.org/10.1080/15226514.2021.1961121
SnehaLatha P, Biftu WK, Suneetha M, Ravindhranath K (2021b) De-fluoridation studies: using Lanthanum-alginate-beads impregnated with green synthesized nSiO2 and active carbon of Terminalia Ivorensis plant as an effective adsorbent. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-021-03575-w
SnehaLatha P, Biftu WK, Suneetha M, Ravindhranath K (2021c) Effective adsorbents based on nano mixed (Al-Fe-Zr) oxide synthesised by new green methods: for the simultaneous extraction of phosphate and chromate from contaminated water. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2021.1927004
Sujitha R, Ravindhranath K (2018) Removal of lead (II) from wastewater using active carbon of Caryota urens seeds and its embedded calcium alginate beads as adsorbents. J Environ Chem Eng 6(4):4298–4309. https://doi.org/10.1016/j.jece.2018.06.033
Sun H, He X, Wang Y, Cannon FS, Wen H, Li X (2018) Nitric acid-anionic surfactant modified activated carbon to enhance cadmium(II) removal from wastewater: preparation conditions and physicochemical properties. Water Sci Technol 78:1489–1498
Suneetha M, Ravindhranath K (2018) Removal of Nitrites from polluted waters using adsorbents derived from Phyllanthus neruri plant. Indian J Chem Technol 25:345–352
Suneetha M, Ravindhranath K (2017) Adsorption of Nitrite ions from wastewater using bio-sorbents derived from Azadirachta indica plant. Asian J Water Environ Pollut 14(2):71–79. https://doi.org/10.3233/AJW-170017
Suneetha M, Syama Sundar B, Ravindhranath K (2015a) Removal of fluoride from polluted waters using active carbon derived from barks of Vitex negundo plant. J Anal Sci Technol, 6(15). https://doi.org/10.1186/s40543-014-0042-1
Suneetha M, SyamaSundar B, Ravindhranath K (2015b) De-fluoridation of waters using low-cost HNO3 activated carbon derived from stems of Senna occidentalis plant. Int J Environ Technol Manage 18(5/6):420–447. https://doi.org/10.1504/IJETM.2015.073079
Suneetha M, SyamaSundar B, Ravindhranath K (2015c) Extraction of fluoride from polluted waters using low-cost active carbon derived from stems of Acalypha indica plant. Asian J Water Environ Pollut 12(3):33–49. https://doi.org/10.3233/AJW-150005
Sunil K, Jayant K (2014) Regeneration and recovery in adsorption- a review. Int J Innov Sci Eng Technol 1(8):61–64
Temkin MJ, Pyzhev V (1940) Recent modifications to Langmuir isotherms. Acta Physiochim USSR 12:217–222
Thabede PM, Shooto ND, Naidoo EB (2020) Removal of methylene blue dye and lead ions from aqueous solution using activated carbon from black cumin seeds. S Afr J Chem Eng 33:39–50. https://doi.org/10.1016/j.sajce.2020.04.002
World Health Organization (2011a) Lead in drinking water. Report No. WHO/SDE/WSH/03.04/09/ Rev/1, Geneva
World Health Organization (2011b) Cadmium in drinking water. Report No. WHO/SDE/WSH/03.04/80/ Rev/1, Geneva
Acknowledgements
The authors thank the authorities of K.L.E.F. for providing the necessary facilities for this research work.
Author information
Authors and Affiliations
Contributions
KR: concept formation and guidance during the progress of this research work. SLP and SM: research scholars, collection of bio-materials for identifying the material having affinity for toxic lead and cadmium ions, optimization of extraction conditions, evaluation of results, evaluation of thermodynamic, kinetic and isothermal parameters to assess the mechanism of adsorption, application of the developed methodology for assessing the ability of bio-material to treat real polluted water.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pala, S.L., Mekala, S. & Ravindhranath, K. Novel adsorbents for simultaneous extraction of lead and cadmium ions from polluted water: based on active carbon, nanometal (Zr-Ce-Sm)-mixed oxides and iron-alginate beads. Biomass Conv. Bioref. 14, 10959–10978 (2024). https://doi.org/10.1007/s13399-022-03063-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03063-2