Abstract
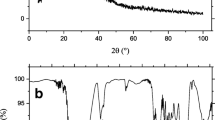
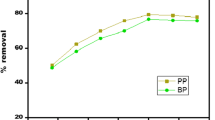
The adsorptive removal of methylene blue (MB) from its aqueous solution using citric acid-modified arjun bark powder as an adsorbent was studied. Initially, the adsorbent was characterized by various analytical techniques (SEM, BET, FTIR, and pHpzc). After characterizing the adsorbent, it was used for carrying out batch study on the biosorption of MB. The batch experiments revealed a very high percentage removal of MB of about 100%. The batch experiment results followed Temkin isotherm. The maximum monolayer adsorption capacity observed was 512.82 mg/g at natural pH of 7.5. The Freundlich isotherm constant was found to be 300.15 l/g. The Temkin isotherm constant was found to be 21.32 l/mg. The sorption energy was calculated from the Dubinin–Radushkevich isotherm model, and it was found to be 10 kJ /mol. On the other hand, results followed the pseudo-second-order kinetic model. The energy of activation in the present study was estimated to be 10 kJ/mol. The adsorptive mass transfer coefficient was calculated to be 1.65 × 10–7 m/s, and the value of diffusivity was found to be 2.27 × 10–11.









Similar content being viewed by others
References
Pigatto RS, Freance DSP, Netto MS, Elvis C, Matias SN, Elvis C, Oliveira LFS, Jahn SL, Dotto GL (2020) An eco-friendly and low-cost strategy for groundwater defluorination: Adsorption of fluoride onto calcinated sludge. J Environ ChemEng 8(6): 104546
Streit AFM, Collazza GC, Druzian SP, Verdi RS, Foletto EL, Oliveira LFS, Dotto GL (2021) Adsorption of ibuprofen, ketoprofen, and paracetamol onto activated carbon prepared from effluent treatment plant sludge of the beverage industry. Chemosphere 262: 128322
Lazarotto JS, Martinello KB, Georgin J, France DSP, Netto MS, Piccilli DGA, Silva LFO, Lima FC, Dotto GL (2021) Preparation of activated carbon from the residues of the mushroom (Agaricusbisporus) production chain for the adsorption of the 2,4-dichlorophenoxyacetic herbicide. J Environ Chem Eng 9:10684
Sheng J, Xie Y, Zhou Y (2009) Adsorption of methylene bluefrom aqueous solution on pyrophyllite. Appl Clay Sci 46:422–430
Hameed BH, Din ATM, Ahmad AL (2007) Adsorption ofmethylene blue onto bamboo-based activated carbon: Kinetics andequilibrium studies. J Hazard Mater 141:819–825
Zhang W, Li H, Kan X, Dong L (2012) Adsorption of anionic dyes from aqueous solutions using chemically modified straw. Bioresour Technol 117:40–47
McKay G, Otterburn SM, Aga AD (1985) Fuller earth and fired clay as adsorbent for dye stuffs. Equilibrium and rate constants. Water Air Soil Pollut 24:307–322
Golob V, VinderA SM (2009) Efficiency of the coagulation flocculation method for the treatment of dye bath effluents. Dyes Pigments 90:2313–2342
Chowdhury A, Sarkar A, Bandyopadhyay A (2009) Rice husk ash as a low cost adsorbent for the removal of methylene blue and congo red in aqueous phases. CLEAN – Soil. Air Water 37:581–591
Yu S, Liu M, Ma M, Qi M, Gao ZIC (2010) Impacts of membrane properties on reactive dye removal from dye/salt mixtures by asymmetric cellulose acetate and composite polyamide nanofiltration membranes. J Mem Sci Technol 350:83–91
Aleboyeh A, Olya ME, Aleboyeh H (2008) Electrical energy determination for an azo dye decolorization and mineralization by UV/H2O2 advanced oxidation process. Chem Eng J 137:518–524
Kerkhoff CM, Boit MKD, Franco DSP, Netto MS, Georgin J, Foletto EL, Piccilli DGA, Silva LFO, Dotto GL (2021) Adsorption of ketoprofen and paracetamol and treatment of a synthetic mixture by novel porous carbon derived from butiacapitata endocarp. J Mol Liq 339: 117184
Sellaoui L, Silva LFO, Badawi M, Ali J, Favarin N, Dotto GL, Erto A, Chen Z (2021) Adsorption of ketoprofen and 2-nitrophenol on activated carbon prepared from winery waste: A combined experimental and theoretical study. J Mol Liq 333: 115906
Georgin J, Franco DSP, Schadeck NM, Allasia D, Foletto EL, Oliveira LFS, Dotto GL (2021) Transforming shrub waste into a high-efficiency adsorbent: Application of physalisperuvian chalice treated with strong acid to remove the 2,4-dichlorophenoxyacetic acid herbicide. J Environ Chem Eng 9: 104574
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: A review. Bioresour Technol 97:1061–1085
Akinyemi SA, Gitari WM, Petrik LF, Oliveira MLS, Silva LFO (2019) Environmental evaluation and nano-mineralogical study of fresh and unsaturated weathered coal fly ashes. Sci Total Environ 663:177–188
Civeira MS, Ramos CG, Oliveira MLS, Kautzmann RM, Taffarel SR, Teixeira EC, Silva LF (2016) Nano-mineralogy of suspended sediment during the beginning of coal rejects spill. Chemosphere 145:142–147
Dalmora AC, Ramos CG, Querol X, Kautzmann RM, Oliveira MLS, Taffarel SR, Moreno T, Silva LF (2016) Nanoparticulate mineral matter from basalt dust wastes. Chemosphere 144:2013–1017
Dutta M, Islam N, Rabha S, Silva LFO, Saikia BK (2020) Acid mine drainage in an india high-sulfur coal mining area: Cytotoxicity assay and remediation study. J Hazard Mater 389: 121851
Islam N, Rabha S, Silva LFO, Saikia BK (2019) Air quality and PM10 associated poly-aromatic hydrocarbons around the railway traffic area: statistical and air mass trajectory approaches. Environ Geochem Health 42(5):2039–2053
Nordin AP, Da Silva J, De Souza C, Niekraszewicz LAB, Dias JF, Da Boit K, Oliveira MLS, Grivicich I, Garcia AL, Silva LF, Da Silva FR (2018) In vitro genotoxic effect of secondary minerals crystallized in rocks from coal mine drainage. J Hazard Mater 346:236–272
Leon-Mejia G, Machado MN, Okuro RT, Silva LF, Telles C, Dias J, Niekraszewicz L, Da Silva J, Henriques JAP, Zin WA (2018) Intratracheal instillation of coal and coal fly ash particles in mice induces DNA damage and translocation of metals to extrapulmonary tissues. Sci Total Environ 625:589–599
Zamberlan DC, Halmenschelager PT, Silva LFO, da Rocha JBT (2020) Copper decreases associative learning and memory in Drosophilia melanogaster. Sci Total Environ 710: 135306
Saikia M, Das T, Dihingia N, Silva LFO, Saikia BK (2020) Formation of carbon quantum dots and grapheme nanosheets from different abundant carbonaceous materials. Diam Relat Mater 106: 107813
Silva LFO, Crissien TJ, Tutikian BF, Sampaio CH (2020b) Rare earth elements and carbon nanotubes in coal mine around spontaneous combustions. J Clean Prod 253: 120068
Uddin MdT, Rukanuzzaman Md, Islam MdA (2009) Adosrption of methylene blue from aqueous solution by jackfruit (Artocarpusheteropyllus) leaf powder: A fixed bed column study. J Environ Manag 90(11):3443–3450
Singh J, Mishra NS, Banerjee S, Sharma YC (2011) Comparative studies of physical characteristics of raw and midified sawdust for their use as adsorbents for removal of acid dye. BioResources 6(3):2732–2743
Mondal D, Goswami A, Purkait MK (2014) Treatment of colored effluent using surfactant modified bamboo leaves powder. Sep Sci Tech 49:221–231
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1368
Weber TW, Chakraborti RK (1947) Pore and solid diffusion models for fixed bed adsorption. AIChE J 20:228–238
Freundlich H (1906) Adsorption in solution. Phy Chem Soc 40:1362–1368
Temkin MI, Pyzhev V (1940) Kinetic of ammonia synthesis on promoted iron catalyst. Acta Physiochim URSS 12:327–356
Halsey G (1948) Physical adsorption on non-uniform surface. J Chem Phys 16:931–937
Dubinin MM (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically non- uniform surface. Chem Rev 60:235–266
Polanyi M (1914) Adsorption from the point of view of the third law of thermodynamics. Verh Deut Phys Ges 16:1012–1016
Yao Z, Wang L, Qi J (2009) Biosorption of methylene blue from aqueous solution using a bio energy forest waste: Xanthocerassorbifolia seed coat. CLEAN-Soil Air Water 37:642–648
Santhi T, Manonmani S, Vasantha VS, Chang YT (2011) A new alternative adsorbent for the removal of cationic dyes from aqueoussolution. Arabian J Chem 2011(06):004
Bestani B, Benderdouche N, Benstaali B, Belhakem M, Addou A (2008) Methylene blue and iodine adsorption onto an activated desert plant. Bioresour Technol 99:8441–8444
Lata H, Garg VK, Gupta RK (2007) Removal of a basic dye from aqueous solution by adsorption using Partheniumhysterophorus: An agricultural waste. Dyes Pigments 74:653–658
Hameed BH, Ahmad AA (2009) Batch adsorption of methyleneblue from aqueous solution by garlic peel, an agricultural waste biomass. J Hazard Mater 164:870–875
Uddin MT, Islam MA, Mahmud S, Rukanuzzaman M (2009) Adsorptive removal of methylene blue by tea waste. J Hazard Mater 164:53–60
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. Kung Sven Veten Hand 24:1–6
Ho SY, McKay G (1999) Pseudo second order model for sorption process. Process Biochem 34:455–465
Namasivayam C, Kavitha D (2002) Removal of congo red from water by adsorption onto activated carbon prepared from coir pith, an agriculture solid waste. Dyes Pigment 161:387–395
Weber WJ, Morris JC (1962) Advances in water pollution research: removal of biologically resistant pollutant from waste water by adsorption, International conference on water pollution symposium. Pergamon Oxford 2:231–266
Boyd GE, Adamson AW, Myers LS (1947) The exchange adsorption of ions from aqueous solutions by organic zeolites. II Kinetics J Am Chem Soc 69:2836–2848
Vermeulen T (1953) Theory for irreversible and constant pattern solid diffusion. Ind Eng Chem 45:1664–1670
Ho YS, Mc Kay G (2002) Application of kinetic models to the sorption of copper (II) onto peat. Adsorpt Sci Technol 20:797–815
Funding
Authors are grateful to the Rashtriya Uchchatar Siksha Abhiyan (RUSA), Ministry of Human Resource Development (MHRD), Govt. of India for their financial aid through research project no. R-11/80/2019.
Author information
Authors and Affiliations
Contributions
SR conceived the project. DKM carried out the experiments. SR and DKM analyzed the data. Both the authors contributed equally for preparation of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roy, S., Mondal, D.K. Parametric Optimization and Kinetics Study of Effective Removal of Methylene Blue by Citric Acid-Modified Arjun Bark Powder. Biomass Conv. Bioref. 14, 1581–1592 (2024). https://doi.org/10.1007/s13399-022-02590-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02590-2