Abstract
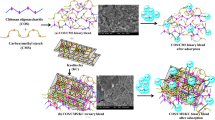
Removal of heavy metal ions from wastewater is of crucial importance for an unpolluted environment and social wellbeing. The present study was designed to prepare a novel copolymeric blend as an efficient adsorbent using chitosan oligosaccharide for the removal of toxic heavy metals Pb and Cd. The ternary blend of Chitosan oligosaccharide (COS) – g – glycidyl methacrylate copolymer (GM)/polypropylene glycol (PPG)/bentonite (Bent) was prepared and the change in morphology was ascertained by FTIR, XRD, TGA, and SEM studies. Using the prepared ternary blend, the adsorption of heavy metals was studied through batch mode to elucidate the changes in adsorption behavior. The removal percentage of lead was 89%, which was higher than cadmium (78%). The adsorption was well fitted with Freundlich isotherm (R2 for Pb: 0.9943 and Cd: 0.9990) and pseudo-second-order kinetics (R2 = Pb: 0.9994 and Cd: 0.9984) with Cmax 177 mg/g (for Pb(II)) and 158 mg/g (for Cd(II)) respectively. Different functional groups which were involved in the binding of the heavy metals were confirmed by SEM-EDAX and FTIR examination. Desorption studies were done using different eluents and the results exposed that the desorbing agent EDTA has great efficiency. The XRD patterns of metal adsorbed ternary blend showed the crystalline phases in their structures concluding the binding of metal ions and the renewal of an amorphous pattern of metal desorbed ternary blend revealed that the desorbed material is suitable for next cycle adsorption.

















Similar content being viewed by others
Data availability
Data is contained within the article. Acknowledgement: One of the authors (Ayman A. Ghfar) is grateful to the Researchers Supporting Project number (RSP-2021/407), King Saud University, Riyadh, Saudi Arabia for the support. The authors acknowledge the support and facilities given by the DST-FIST-LAB, D.K.M. College of Women, Vellore, Tamil Nadu, India.
References
Russo T, Fucile P, Giacometti R, Sannino F (2021) Sustainable removal of contaminants by biopolymers: a novel approach for wastewater treatment. Current State and Future Perspectives. Process. 9
Al-Ghouti MA, Dib SS (2020) Utilization of nano-olive stones in environmental remediation of methylene blue from water 03 Chemical Sciences 0306 Physical Chemistry (incl. Structural). J Environ Heal Sci Eng 18:63–77. https://doi.org/10.1007/s40201-019-00438-y
Barbosa PFP, Cumba LR, Andrade RDA, do Carmo DR (2019) Chemical modifications of cyclodextrin and chitosan for biological and environmental applications: metals and organic pollutants adsorption and removal. J Polym Environ 27:1352–1366. https://doi.org/10.1007/s10924-019-01434-x
Zhang S, Du Q, Sun Y et al (2020) Fabrication of L-cysteine stabilized α-FeOOH nanocomposite on porous hydrophilic biochar as an effective adsorbent for Pb2+ removal. Sci Total Environ 720:137415. https://doi.org/10.1016/j.scitotenv.2020.137415
Kumar M, Chung JS, Hur SH (2019) Graphene composites for lead ions removal from aqueous solutions. Appl Sci 9. https://doi.org/10.3390/app9142925
Abdel-Shafy HI, Mansour MSM (2018) Solid waste issue: sources, composition, disposal, recycling, and valorization. Egypt J Pet 27:1275–1290. https://doi.org/10.1016/j.ejpe.2018.07.003
Meng Q-Y, Wang H, Cui Z-B et al (2020) Chitosan oligosaccharides attenuate amyloid formation of hIAPP and protect pancreatic β-cells from cytotoxicity. Molecules 25:1314. https://doi.org/10.3390/molecules25061314
Yue L, Sun D, Mahmood Khan I et al (2020) Cinnamyl alcohol modified chitosan oligosaccharide for enhancing antimicrobial activity. Food Chem 309:125513. https://doi.org/10.1016/j.foodchem.2019.125513
Lee HW, Karim MR, Park JH et al (2009) Poly(vinyl alcohol)/chitosan oligosaccharide blend submicrometer fibers prepared from aqueous solutions by the electrospinning method. J Appl Polym Sci 111:132–140. https://doi.org/10.1002/app.29033
Ajitha P, Vijayalakshmi K, Saranya M et al (2017) Removal of toxic heavy metal lead (II) using chitosan oligosaccharide-graft-maleic anhydride/polyvinyl alcohol/silk fibroin composite. Int J Biol Macromol 104:1469–1482. https://doi.org/10.1016/j.ijbiomac.2017.05.111
Chen Y, Zhao W, Wang H et al (2021) A novel polyamine-type starch/glycidyl methacrylate copolymer for adsorption of Pb(II), Cu(II), Cd(II) and Cr(III) ions from aqueous solutions. R Soc Open Sci 5:180281. https://doi.org/10.1098/rsos.180281
Rajan M, Praphakar RA, Govindaraj D et al (2017) Cytotoxicity assessment of palbociclib-loaded chitosan-polypropylene glycol nano vehicles for cancer chemotherapy. Mater Today Chem 6:26–33. https://doi.org/10.1016/j.mtchem.2017.08.002
Garba ZN, Zhou W, Zhang M, Yuan Z (2020) A review on the preparation, characterization and potential application of perovskites as adsorbents for wastewater treatment. Chemosphere 244:125474. https://doi.org/10.1016/j.chemosphere.2019.125474
Zou C, Liang J, Jiang W et al (2018) Adsorption behavior of magnetic bentonite for removing Hg(ii) from aqueous solutions. RSC Adv 8:27587–27595. https://doi.org/10.1039/C8RA05247F
Raafat D, Sahl H-G (2009) Chitosan and its antimicrobial potential–a critical literature survey. Microb Biotechnol 2:186–201. https://doi.org/10.1111/j.1751-7915.2008.00080.x
Shukla SR, Athalye AR (1994) Graft-copolymerization of glycidyl methacrylate onto cotton cellulose. J Appl Polym Sci 54:279–288. https://doi.org/10.1002/app.1994.070540302
Irshad H, Khalid N, Rubab Z, et al (2018) Study of biodegradability by graft copolymerization of starch backbone with acrylic acid , methacrylate , acetonitrile with the advancement of Ni-doped nanoparticles. 9:47–55
Tsai C-H, Chang W-C, Saikia D et al (2016) Functionalization of cubic mesoporous silica SBA-16 with carboxylic acid via one-pot synthesis route for effective removal of cationic dyes. J Hazard Mater 309:236–248. https://doi.org/10.1016/j.jhazmat.2015.08.051
Dotto GL, Rodrigues FK, Tanabe EH et al (2016) Development of chitosan/bentonite hybrid composite to remove hazardous anionic and cationic dyes from colored effluents. J Environ Chem Eng 4:3230–3239. https://doi.org/10.1016/j.jece.2016.07.004
de Luna MDG, Futalan CM, Jurado CA Jr et al (2018) Removal of ammonium-nitrogen from aqueous solution using chitosan-coated bentonite: mechanism and effect of operating parameters. J Appl Polym Sci 135:45924. https://doi.org/10.1002/app.45924
Gaouar Yadi M, Benguella B, Gaouar-Benyelles N, Tizaoui K (2016) Adsorption of ammonia from wastewater using low-cost bentonite/chitosan beads. Desalin Water Treat 57:21444–21454. https://doi.org/10.1080/19443994.2015.1119747
Rahman A ur, Iqbal M, Rahman F ur, et al (2012) Synthesis and characterization of reactive macroporous poly(glycidyl methacrylate-triallyl isocyanurate-ethylene glycol dimethacrylate) microspheres by suspension polymerization: Effect of synthesis variables on surface area and porosity. J Appl Polym Sci 124:915–926. https://doi.org/10.1002/app.35026
Grochowicz M, Pączkowski P, Gawdzik B (2018) Investigation of the thermal properties of glycidyl methacrylate–ethylene glycol dimethacrylate copolymeric microspheres modified by Diels-Alder reaction. J Therm Anal Calorim 133:499–508. https://doi.org/10.1007/s10973-017-6785-3
Roca Jalil ME, Vieira RS, Azevedo D et al (2013) Improvement in the adsorption of thiabendazole by using aluminum pillared clays. Appl Clay Sci 71:55–63. https://doi.org/10.1016/j.clay.2012.11.005
Sarı Yılmaz M, Kalpaklı Y, Pişkin S (2013) Thermal behavior and dehydroxylation kinetics of naturally occurring sepiolite and bentonite. J Therm Anal Calorim 114:1191–1199. https://doi.org/10.1007/s10973-013-3152-x
Viciosa MT, Dionı́sio M (2004) Molecular mobility and fragility in n-ethylene glycol dimethacrylate monomers. J Non Cryst Solids 341:60–67. https://doi.org/10.1016/j.jnoncrysol.2004.04.018
Lfa AR, Jeslin J (2018) Synthesis of bentonite nanoclay and incorporation of cassia fistula leaf extract to form organobentonite: characterization and its biomedical applications. Asian J Pharm Clin Res 11:381–384. https://doi.org/10.22159/ajpcr.2018.v11i9.26717
Vilvanathan S, Shanthakumar S (2018) Ni2+ and Co2+ adsorption using Tectona grandis biochar: kinetics, equilibrium and desorption studies. Environ Technol 39:464–478. https://doi.org/10.1080/09593330.2017.1304454
Wang H, Yuan X, Wu Y et al (2013) Adsorption characteristics and behaviors of graphene oxide for Zn(II) removal from aqueous solution. Appl Surf Sci 279:432–440. https://doi.org/10.1016/j.apsusc.2013.04.133
Shi J, Zhao Z, Liang Z, Sun T (2016) Adsorption characteristics of Pb(II) from aqueous solutions onto a natural biosorbent, fallen arborvitae leaves. Water Sci Technol 73(10):2422–2429
Tanzifi M, Kolbadi nezhad M, Karimipour K (2017) Kinetic and isotherm studies of cadmium adsorption on polypyrrole/titanium dioxide nanocomposite. J Water Environ Nanotechnol 2:265–277. https://doi.org/10.22090/jwent.2017.04.004
Awual MR, Hasan MM, Eldesoky GE et al (2016) Facile mercury detection and removal from aqueous media involving ligand impregnated conjugate nanomaterials. Chem Eng J 290:243–251. https://doi.org/10.1016/j.cej.2016.01.038
Mi X, Huang G, Xie W et al (2012) Preparation of graphene oxide aerogel and its adsorption for Cu2+ ions. Carbon N Y 50:4856–4864. https://doi.org/10.1016/j.carbon.2012.06.013
Manzoor K, Ahmad M, Ahmad S, Ikram S (2019) Removal of Pb(ii) and Cd(ii) from wastewater using arginine cross-linked chitosan–carboxymethyl cellulose beads as green adsorbent. RSC Adv 9:7890–7902. https://doi.org/10.1039/C9RA00356H
Akter N, Hossain MA, Hassan MJ et al (2016) Amine modified tannin gel for adsorptive removal of Brilliant Green dye. J Environ Chem Eng 4:1231–1241. https://doi.org/10.1016/j.jece.2016.01.013
Shabiimam M. A. KMRHRA (2017) Adsorption of lead by bentonite clay. Int J Sci Res Manag 5:5800–5804. https://doi.org/10.18535/ijsrm/v5i7.02
Putro JN, Santoso SP, Ismadji S, Ju Y-H (2017) Investigation of heavy metal adsorption in binary system by nanocrystalline cellulose – bentonite nanocomposite: improvement on extended Langmuir isotherm model. Microporous Mesoporous Mater 246:166–177. https://doi.org/10.1016/j.micromeso.2017.03.032
Da̧browski A (2001) Adsorption - from theory to practice. Adv Colloid Interface Sci 93:135–224. https://doi.org/10.1016/S0001-8686(00)00082-8
Desta MB (2013) Batch sorption experiments: Langmuir and Freundlich isotherm studies for the adsorption of textile metal ions onto teff straw (Eragrostis tef) agricultural waste. J Thermodyn 2013:375830. https://doi.org/10.1155/2013/375830
Deng L, Su Y, Su H et al (2007) Sorption and desorption of lead (II) from wastewater by green algae Cladophora fascicularis. J Hazard Mater 143:220–225. https://doi.org/10.1016/j.jhazmat.2006.09.009
Meléndez-Ortiz HI, Puente-Urbina B, Mercado-Silva JA, García-Uriostegui L (2019) Adsorption performance of mesoporous silicas towards a cationic dye. Influence of mesostructure on adsorption capacity. Int J Appl Ceram Technol 16:1533–1543. https://doi.org/10.1111/ijac.13179
Pavasant P, Apiratikul R, Sungkhum V et al (2006) Biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga Caulerpa lentillifera. Bioresour Technol 97:2321–2329. https://doi.org/10.1016/j.biortech.2005.10.032
Kilislioglu A, Bilgin B (2003) Thermodynamic and kinetic investigations of uranium adsorption on amberlite IR-118H resin. Appl Radiat Isot 58:155–160. https://doi.org/10.1016/s0969-8043(02)00316-0
Bakir A, McLoughlin P, Tofail SAM, Fitzgerald E (2009) Competitive sorption of antimony with zinc, nickel, and aluminum in a seaweed based fixed-bed sorption column. CLEAN – Soil, Air, Water 37:712–719. https://doi.org/10.1002/clen.200900164
Huo Y, Wu H, Wang Z et al (2018) Preparation of core/shell nanocomposite adsorbents based on amine polymer-modified magnetic materials for the efficient adsorption of anionic dyes. Colloids Surfaces A Physicochem Eng Asp 549:174–183. https://doi.org/10.1016/j.colsurfa.2018.04.021
Kumar ASK, Barathi M, Puvvada S, Rajesh N (2013) Microwave assisted preparation of glycidyl methacrylate grafted cellulose adsorbent for the effective adsorption of mercury from a coal fly ash sample. J Environ Chem Eng 1:1359–1367. https://doi.org/10.1016/j.jece.2013.10.004
M. S, Srinivasan L, M.R. GR, et al (2017) Adsorption studies of lead(II) from aqueous solution onto Nanochitosan /polyurethane /polypropylene glycol ternary blends. Int J Biol Macromol 104:1436–1448. https://doi.org/10.1016/j.ijbiomac.2017.06.004
Jain SN, Gogate PR (2017) Adsorptive removal of acid violet 17 dye from wastewater using biosorbent obtained from NaOH and H2SO4 activation of fallen leaves of Ficus racemosa. J Mol Liq 243:132–143. https://doi.org/10.1016/j.molliq.2017.08.009
Zhu H-Y, Jiang R, Xiao L (2010) Adsorption of an anionic azo dye by chitosan/kaolin/γ-Fe2O3 composites. Appl Clay Sci 48:522–526. https://doi.org/10.1016/j.clay.2010.02.003
de Araujo ALP, Bertagnolli C, da Silva MGC et al (2013) Adsorção de zinco em argila bentonita: influência do pH, quantidade de adsorvente e concentração inicial. Acta Sci - Technol 35:325–332. https://doi.org/10.4025/actascitechnol.v35i2.13364
Bojemueller E, Nennemann A, Lagaly G (2001) Enhanced pesticide adsorption by thermally modified bentonites. Appl Clay Sci 18:277–284. https://doi.org/10.1016/S0169-1317(01)00027-8
Lata S, Singh PK, Samadder SR (2015) Regeneration of adsorbents and recovery of heavy metals: a review. Int J Environ Sci Technol 12:1461–1478. https://doi.org/10.1007/s13762-014-0714-9
Ghasemzadeh N, Ghadiri M, Behroozsarand A (2017) Optimization of chemical regeneration procedures of spent activated carbon. Adv Environ Technol 3:45–51. https://doi.org/10.22104/aet.2017.504
Kordialik-Bogacka E (2011) Cadmium and lead recovery from yeast biomass. Cent Eur J Chem 9:320–325. https://doi.org/10.2478/s11532-011-0001-2
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Radha, E., Gomathi, T., Sudha, P.N. et al. Adsorption studies on removal of Pb(II) and Cd(II) ions using chitosan derived copoymeric blend. Biomass Conv. Bioref. (2021). https://doi.org/10.1007/s13399-021-01918-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-021-01918-8