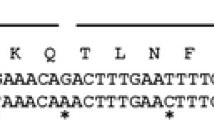
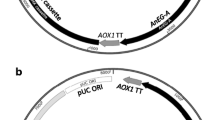
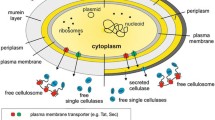
Abstract
Cellulases are among the most important groups of industrial enzymes that are widely consumed in biofuel production, pulp and paper, textile, and detergent industries. The methylotrophic yeast Pichia pastoris was used for heterologous expression of a thermophilic cellulase collection. P. pastoris cells were transformed by the codon-optimized polycistronic EBG construct. This construct included egxA gene (from Ampullaria crossean, with endo- and exoglucanase activities), cglT gene (from Thermoanaerobacter brockii, with β-glucosidase activity), and zsgreen (a fluorescent marker). Gene expression was examined at mRNA level using RT-PCR technique. The results indicated successful transcription of all transgenes. CglT and ZsGreen recombinant proteins were respectively detected by enzymatic assay and fluorescent microscope, while endo- and exoglucanase activities were not determined by enzymatic assays. The highest β-glucosidase activity was measured at 65 ºC and pH 5.5. CglT is a good candidate for completing cellulase collections with low β-glucosidase activity. These cellulase sets could be used in biofuel production because of the high glucose tolerance property of CglT.








Similar content being viewed by others
References
Béguin P, Aubert JP (1994) The biological degradation of cellulose. FEMS Microbiol Rev 13(1):25–58. https://doi.org/10.1111/j.1574-6976.1994.tb00033.x
Ando S, Ishida H, Kosugi Y, Ishikawa K (2002) Hyperthermostable endoglucanase from Pyrococcus horikoshii. Appl Environ Microbiol 68(1):430–433. https://doi.org/10.1128/AEM.68.1.430-433.2002
Dhiman T, Zaman M, Gimenez R, Walters J, Treacher R (2002) Performance of dairy cows fed forage treated with fibrolytic enzymes prior to feeding. Anim Feed Sci Technol 101(1–4):115–125. https://doi.org/10.1016/S0377-8401(02)00177-3
Olofsson K, Bertilsson M, Lidén G (2008) A short review on SSF–an interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol Biofuels 1(1):7. https://doi.org/10.1186/1754-6834-1-7
Baffi MA, Tobal T, Lago JHG, Boscolo M, Gomes E, Da-Silva R (2013) Wine aroma improvement using a β-glucosidase preparation from Aureobasidium pullulans. Appl Biochem Biotechnol 169(2):493–501. https://doi.org/10.1007/s12010-012-9991-2
Bayer EA, Lamed R, Himmel ME (2007) The potential of cellulases and cellulosomes for cellulosic waste management. Curr Opin Biotechnol 18(3):237–245. https://doi.org/10.1016/j.copbio.2007.04.004
Menetrez MY (2012) An overview of algae biofuel production and potential environmental impact. Environ Sci Technol 46(13):7073–7085. https://doi.org/10.1021/es300917r
Pandey K, Singh B, Pandey AK, Badruddin IJ, Pandey S, Mishra VK et al (2017) Application of microbial enzymes in industrial waste water treatment. Int J Curr Microbiol App Sci 6(8):1243–1254. https://doi.org/10.20546/ijcmas.2017.608.151
Kumar S, Nussinov R (2001) How do thermophilic proteins deal with heat? Cell Mol Life Sci 58(9):1216–1233. https://doi.org/10.1007/PL00000935
Ding M, Teng Y, Yin Q, Zhao J, Zhao F (2008) The N‐terminal cellulose‐binding domain of EGXA increases thermal stability of xylanase and changes its specific activities on different substrates (2008). Acta Biochim Biophys Sin 40(11):949–954. https://doi.org/10.1111/j.1745-7270.2008.00481.x
Breves R, Bronnenmeier K, Wild N, Lottspeich F, Staudenbauer WL, Hofemeister J (1997) Genes encoding two different β-glucosidases of Thermoanaerobacter brockii are clustered in a common operon. Appl Environ Microbiol 63(10):3902–3910. https://doi.org/10.1128/AEM.63.10.3902-3910.1997
Cregg JM, Vedvick TS, Raschke WC (1993) Recent advances in the expression of foreign genes in Pichia pastoris. Nat Biotechnol 11(8):905. https://doi.org/10.1038/nbt0893-905
Ahmad M, Hirz M, Pichler H, Schwab H (2014) Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl Microbiol Biotechnol 98(12):5301–5317
Mellitzer A, Weis R, Glieder A, Flicker K (2012) Expression of lignocellulolytic enzymes in Pichia pastoris. Microb Cell Factories 11(1):61. https://doi.org/10.1007/s00253-014-5732-5
Ryan MD, King AM, Thomas GP (1991) Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J Gen Virol 72(11):2727–2732. https://doi.org/10.1099/0022-1317-72-11-2727
Donnelly ML, Hughes LE, Luke G, Mendoza H, Ten Dam E, Gani D, Ryan MD (2001) The ‘cleavage’activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring ‘2A-like’sequences. J Gen Virol 82(5):1027–1041. https://doi.org/10.1099/0022-1317-82-5-1027
de Felipe P, Luke GA, Hughes LE, Gani D, Halpin C, Ryan MD (2006) E unum pluribus: multiple proteins from a self-processing polyprotein. Trends Biotechnol 24(2):68–75. https://doi.org/10.1016/j.tibtech.2005.12.006
Subramanian V, Schuster LA, Moore KT, Taylor LE, Baker JO, Vander Wall TA, Linger JG, Himmel ME, Decker SR (2017) A versatile 2A peptide-based bicistronic protein expressing platform for the industrial cellulase producing fungus Trichoderma reesei. Biotechnol Biofuels 10(1):1–15. https://doi.org/10.1186/s13068-017-0710-7
Liu Z, Chen O, Wall JBJ, Zheng M, Zhou Y, Wang L, Vaseghi HR, Qian L, Liu J (2017) Systematic comparison of 2A peptides for cloning multi-genes in a polycistronic vector. Sci Rep 7(1):1–9. https://doi.org/10.1038/s41598-017-02460-2
Jiao X, Sun W, Zhang Y, Liu X, Zhang Q, Wang Q, Zhang S, Zhao ZK (2018) Exchanging the order of carotenogenic genes linked by porcine teschovirus-1 2A peptide enable to optimize carotenoid metabolic pathway in Saccharomyces cerevisiae. RSC Adv 8(61):34967–34972. https://doi.org/10.1039/c8ra06510a
Geier M, Fauland P, Vogl T, Glieder A (2015) Compact multi-enzyme pathways in P. pastoris. Chem Comm 51(9):1643–1646. https://doi.org/10.1039/c4cc08502g
Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF et al (2004) Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide–based retroviral vector. Nat Biotechnol 22(5):589–594. https://doi.org/10.1038/nbt957
Xiong AS, Yao QH, Peng RH, Han PL, Cheng ZM, Li Y (2005) High level expression of a recombinant acid phytase gene in Pichia pastoris. J Appl Microbiol 98(2):418–428. https://doi.org/10.1111/j.1365-2672.2004.02476.x
Green M, Sambrook J (2012) Molecular cloning: a laboratory manual, 4th edn. Cold Spring Harbor Laboratory Press, New York
Inoue H, Nojima H, Okayama H (1990) High efficiency transformation of Escherichia coli with plasmids. Gene 96(1):23–28. https://doi.org/10.1016/0378-1119(90)90336-p
Burgess RR (2009) Protein precipitation techniques. Methods Enzymol 463:331–342. https://doi.org/10.1016/S0076-6879(09)63020-2
Çağlayan M, Wilson SH (2014) Enzymatic activity assays in yeast cell extracts. Bio-protocol 4(23):e1312. https://doi.org/10.21769/BioProtoc.1312
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428. https://doi.org/10.1021/ac60147a030
Zhang YP, Hong J, Ye X (2009) Cellulase assays. Methods Mol Biol 581:213–231. https://doi.org/10.1007/978-1-60761-214-8_14
Kim YK, Lee SC, Cho YY, Oh HJ, Ko YH (2012) Isolation of cellulolytic Bacillus subtilis strains from agricultural environments. ISRN Microbiol 2012. https://doi.org/10.5402/2012/650563
Deshpande MV, Eriksson KE, Pettersson LG (1984) An assay for selective determination of exo-1, 4,-β-glucanases in a mixture of cellulolytic enzymes. Anal Biochem 138(2):481–487. https://doi.org/10.1016/0003-2697(84)90843-1
Simpson RJ (2006) SDS-PAGE of proteins. Cold Spring Harb Protoc. https://doi.org/10.1101/pdb.prot4313
Brunelle JL, Green R (2014) Coomassie blue staining. Methods Enzymol 541:161–167. https://doi.org/10.1016/B978-0-12-420119-4.00013-6
Bader O, Krauke Y, Hube B (2008) Processing of predicted substrates of fungal Kex2 proteinases from Candida albicans, C. glabrata, Saccharomyces cerevisiae and Pichia pastoris. BMC Microbiol 8(1):1–16. https://doi.org/10.1186/1471-2180-8-116
Bhat M (2000) Cellulases and related enzymes in biotechnology. Biotechnol Adv 18(5):355–383. https://doi.org/10.1016/s0734-9750(00)00041-0
Phitsuwan P, Laohakunjit N, Kerdchoechuen O, Kyu KL, Ratanakhanokchai K (2013) Present and potential applications of cellulases in agriculture, biotechnology, and bioenergy. Folia Microbiol 58(2):163–176. https://doi.org/10.1007/s12223-012-0184-8
Wilson DB (2009) Cellulases and biofuels. Curr Opin Biotechnol 20(3):295–299. https://doi.org/10.1016/j.copbio.2009.05.007
Mazzoli R, Lamberti C, Pessione E (2012) Engineering new metabolic capabilities in bacteria: lessons from recombinant cellulolytic strategies. Trends Biotechnol 30(2):111–119. https://doi.org/10.1016/j.tibtech.2011.08.003
Tsai SL, Oh J, Singh S, Chen R, Chen W (2009) Functional assembly of minicellulosomes on the Saccharomyces cerevisiae cell surface for cellulose hydrolysis and ethanol production. Appl Environ Microbiol 75(19):6087–6093. https://doi.org/10.1128/AEM.01538-09
Waeonukul R, Kosugi A, Tachaapaikoon C, Pason P, Ratanakhanokchai K, Prawitwong P et al (2012) Efficient saccharification of ammonia soaked rice straw by combination of Clostridium thermocellum cellulosome and Thermoanaerobacter brockii β-glucosidase. Bioresour Technol 107:352–357. https://doi.org/10.1016/j.biortech.2011.12.126
Geng A, Wu J, Xie R, Li X, Chang F, Sun J (2015) Construction of a bacterial cellulase cocktail for saccharification of regenerated cellulose and pretreated corn stover. BioResources 10(4):7681–92. https://doi.org/10.15376/biores.10.4.7681-7692
Gueguen Y, Chemardin P, Janbon G, Arnaud A, Galzy P (1998) Investigation of the β-glucosidases potentialities of yeast strains and application to bound aromatic terpenols liberation. Stud Org Chem 53:149–157. https://doi.org/10.1016/S0165-3253(98)80018-7
Gupta A, Kumar V, Dubey A, Verma A (2014) Kinetic characterization and effect of immobilized thermostable β-glucosidase in alginate gel beads on sugarcane juice. ISRN Biochem 2014.https://doi.org/10.1155/2014/178498
Su E, Xia T, Gao L, Dai Q, Zhang Z (2010) Immobilization of β-glucosidase and its aroma-increasing effect on tea beverage. Food Bioprod Process 88(2):83–89. https://doi.org/10.1016/j.fbp.2009.04.001
Hu SC, Hong K, Song YC, Liu JY, Tan RX (2009) Biotransformation of soybean isoflavones by a marine Streptomyces sp. 060524 and cytotoxicity of the products. World J Microbiol Biotechnol 25(1):115. https://doi.org/10.1007/s11274-008-9872-6
Pandjaitan N, Hettiarachchy N, Ju Z (2000) Enrichment of genistein in soy protein concentrate with β-glucosidase. J Food Sci 65(3):403–407. https://doi.org/10.1111/j.1365-2621.2000.tb16055.x
Coenen T, Schoenmakers A, Verhagen H (1995) Safety evaluation of β-glucanase derived from Trichoderma reesei: summary of toxicological data. Food Chem Toxicol 33(10):859–866. https://doi.org/10.1016/0278-6915(95)00052-4
Zhang Z, Marquardt RR, Wang G, Guenter W, Crow GH, Han Z et al (1996) A simple model for predicting the response of chicks to dietary enzyme supplementation. J Anim Sci 74(2):394–402. https://doi.org/10.2527/1996.742394x
Aggarwal S, Mishra S (2020) Differential role of segments of α-mating factor secretion signal in Pichia pastoris towards granulocyte colony-stimulating factor emerging from a wild type or codon optimized copy of the gene. Microb Cell Fact 19(1):1–16. https://doi.org/10.1186/s12934-020-01460-8
Rotticci-Mulder JC, Gustavsson M, Holmquist M, Hult K, Martinelle M (2001) Expression in Pichia pastoris of Candida antarctica lipase B and lipase B fused to a cellulose-binding domain. Protein Expr Purif 21(3):386–392. https://doi.org/10.1006/prep.2000.1387
Camarero S, Pardo I, Cañas AI, Molina P, Record E, Martínez A, Martínez MJ, Alcalde M (2012) Engineering platforms for directed evolution of laccase from Pycnoporus cinnabarinus. Appl Environ Microbiol 78(5):1370–1384. https://doi.org/10.1128/AEM.07530-11
Ardila-Leal LD, Alvarado-Ramírez MF, Gutiérrez-Rojas IS, Poutou-Pinales RA, Quevedo-Hidalgo B, Pérez-Flórez A, Pedroza-Rodríguez AM (2020) Low-cost media statistical design for laccase rPOXA 1B production in P. pastoris. Heliyon 6(4):e03852. https://doi.org/10.1016/j.heliyon.2020.e03852
Fitzgerald I, Glick BS (2014) Secretion of a foreign protein from budding yeasts is enhanced by cotranslational translocation and by suppression of vacuolar targeting. Microb Cell Fact 13(1):125. https://doi.org/10.1186/s12934-014-0125-0
Kjeldsen T, Ludvigsen S, Diers I, Balschmidt P, Sørensen AR, Kaarsholm NC (2002) Engineering-enhanced protein secretory expression in yeast with application to insulin. J Biol Chem 277(21):18245–18248. https://doi.org/10.1074/jbc.C200137200
Li J, Sun C, Chen L, Sun L, Duan L, Zheng Q, Hu X (2017) Optimization of the secretory expression of recombinant human C-reactive protein in Pichia pastoris. 3 Biotech 7(5):1–8. https://doi.org/10.1007/s13205-017-0917-0
Fuller RS, Sterne RE, Thorner J (1988) Enzymes required for yeast prohormone processing. Annu Rev Physiol 50(1):345–362. https://doi.org/10.1146/annurev.ph.50.030188.002021
Waters MG, Evans EA, Blobel G (1988) Prepro-alpha-factor has a cleavable signal sequence. J Biol Chem 263(13):6209–6214. https://doi.org/10.1016/S0021-9258(18)68773-3
Paetzel M, Karla A, Strynadka NC, Dalbey RE (2002) Signal peptidases. Chem Rev 102(12):4549–4580. https://doi.org/10.1021/cr010166y
Singh A, Lugovoy JM, Kohr WJ, Perry LJ (1984) Synthesis, secretion and processing of α-factor-interferon fusion proteins Id yeast. Nucleic Acids Res 12(23):8927–8938. https://doi.org/10.1093/nar/12.23.8927
Germain D, Dumas F, Vernet T, Bourbonnais Y, Thomas DY, Boileau G (1992) The pro-region of the Kex2 endoprotease of Saccharomyces cerevisiae is removed by self-processing. FEBS Lett 299(3):283–286. https://doi.org/10.1016/0014-5793(92)80132-z
Julius D, Blair L, Brake A, Sprague G, Thorner J (1983) Yeast α factor is processed from a larger precursor polypeptide: the essential role of a membrane-bound dipeptidyl aminopeptidase. Cell 32(3):839–852. https://doi.org/10.1016/0092-8674(83)90070-3
Ng DT, Brown JD, Walter P (1996) Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J Cell Biol 134(2):269–278. https://doi.org/10.1083/jcb.134.2.269
Plath K, Mothes W, Wilkinson BM, Stirling CJ, Rapoport TA (1998) Signal sequence recognition in posttranslational protein transport across the yeast ER membrane. Cell 94(6):795–807. https://doi.org/10.1016/s0092-8674(00)81738-9
Ngosuwan J, Wang NM, Fung KL, Chirico WJ (2003) Roles of cytosolic Hsp70 and Hsp40 molecular chaperones in post-translational translocation of presecretory proteins into the endoplasmic reticulum. J Biol Chem 278(9):7034–7042. https://doi.org/10.1074/jbc.M210544200
Barrero JJ, Casler JC, Valero F, Ferrer P, Glick BS (2018) An improved secretion signal enhances the secretion of model proteins from Pichia pastoris. Microb Cell Fact 17(1):1–13. https://doi.org/10.1186/s12934-018-1009-5
Finger A, Knop M, Wolf DH (1993) Analysis of two mutated vacuolar proteins reveals a degradation pathway in the endoplasmic reticulum or a related compartment of yeast. FEBS J 218(2):565–574. https://doi.org/10.1111/j.1432-1033.1993.tb18410.x
Kang HA, Lee KN, Yu MH (1997) Folding and stability of the Z and Siiyama genetic variants of human α1-antitrypsin. J Biol Chem 272(1):510–516. https://doi.org/10.1074/jbc.272.1.510
Hiller MM, Finger A, Schweiger M, Wolf DH (1996) ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science 273(5282):1725. https://doi.org/10.1126/science.273.5282.1725
Werner ED, Brodsky JL, McCracken AA (1996) Proteasome-dependent endoplasmic reticulum-associated protein degradation: an unconventional route to a familiar fate. Proc Natl Acad Sci 93(24):13797–13801. https://doi.org/10.1073/pnas.93.24.13797
Hong E, Davidson AR, Kaiser CA (1996) A pathway for targeting soluble misfolded proteins to the yeast vacuole. J Cell Biol 135(3):623–633. https://doi.org/10.1083/jcb.135.3.623
Holkeri H, Makarow M (1998) Different degradation pathways for heterologous glycoproteins in yeast. FEBS Lett 429(2):162–166. https://doi.org/10.1016/s0014-5793(98)00586-9
Jørgensen MU, Emr SD, Winther JR (1999) Ligand recognition and domain structure of Vps10p, a vacuolar protein sorting receptor in Saccharomyces cerevisiae. FEBS J 260(2):461–469. https://doi.org/10.1046/j.1432-1327.1999.00176.x
Zhang BY, Chang A, Kjeldsen TB, Arvan P (2001) Intracellular retention of newly synthesized insulin in yeast is caused by endoproteolytic processing in the Golgi complex. J Cell Biol 153(6):1187–1198. https://doi.org/10.1083/jcb.153.6.1187
Rakestraw JA, Sazinsky SL, Piatesi A, Antipov E, Wittrup KD (2009) Directed evolution of a secretory leader for the improved expression of heterologous proteins and full-length antibodies in Saccharomyces cerevisiae. Biotechnol Bioeng 103(6):1192–1201. https://doi.org/10.1002/bit.22338
Wang TY, Huang CJ, Chen HL, Ho PC, Ke HM, Cho HY et al (2013) Systematic screening of glycosylation-and trafficking-associated gene knockouts in Saccharomyces cerevisiae identifies mutants with improved heterologous exocellulase activity and host secretion. BMC Biotechnol 13(1):71. https://doi.org/10.1186/1472-6750-13-71
Marcusson EG, Horazdovsky BF, Cereghino JL, Gharakhanian E, Emr SD (1994) The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell 77(4):579–586. https://doi.org/10.1016/0092-8674(94)90219-4
Brake AJ (1990) α-Factor leader-directed secretion of heterologous proteins from yeast. Methods Enzymol 185:408–421. https://doi.org/10.1016/0076-6879(90)85036-n
Brenner C, Fuller RS (1992) Structural and enzymatic characterization of a purified prohormone-processing enzyme: secreted, soluble Kex2 protease. Proc Natl Acad Sci USA 89(3):922–926. https://doi.org/10.1073/pnas.89.3.922
Cawley NX, Olsen V, Zhang CF, Chen HC, Tan M, Loh YP (1998) Activation and processing of non-anchored yapsin 1 (Yap3p). J Biol Chem 273(1):584–591. https://doi.org/10.1074/jbc.273.1.584
Xie YF, Chen H, Huang BR (2007) Expression, purification and characterization of human IFN-λ1 in Pichia pastoris. J Biotechnol 129(3):472–480. https://doi.org/10.1016/j.jbiotec.2007.01.018
Ding M, Teng Y, Yin Q, Zhao J, Zhao F (2008) The N-terminal cellulose-binding domain of EGXA increases thermal stability of xylanase and changes its specific activities on different substrates. Acta Biochim Biophys Sin 40(11):949–954. https://doi.org/10.1111/j.1745-7270.2008.00481.x
Liu Z, Sun Y, Feng T, Ji Q, Cong P, Chen Y, He Z (2014) Mammalian expression levels of cellulase and xylanase genes optimised by human codon usage are not necessarily higher than those optimised by the extremely biased approach. Biotechnol Lett 36(11):2169–2176. https://doi.org/10.1007/s10529-014-1592-4
Acknowledgements
We would like to appreciate Dr. Moein Farshchian for his assistance in gene construct design.
Funding
This work was supported by the Biotechnology Development Council (grant number, 100485) and Ferdowsi University of Mashhad (grant number, 29524).
Author information
Authors and Affiliations
Contributions
Conceptualization: Bahrami, Matin, Javanmard
Methodology: Javanmard
Data analysis: Javanmard
Writing—original draft preparation: Javanmard
Writing—review, and editing: Bahrami, Matin
Supervision: Bahrami, Matin
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Javanmard, A.S., Matin, M. & Bahrami, A.R. Polycistronic cellulase gene expression in Pichia pastoris. Biomass Conv. Bioref. 13, 7151–7163 (2023). https://doi.org/10.1007/s13399-021-01765-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01765-7