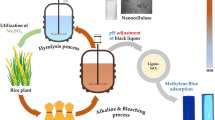
Abstract
The conversion of rice crop residues into activated carbon (AC) and nanosilica was performed via an integrated procedure including successive carbonization, K2CO3 activation, and extraction steps. For the first time, rice husk and rice straw were utilized comparatively as the raw materials for parallel AC and silica production, and the main characteristics of the corresponding products were compared. However, AC produced from rice straw at 1000 °C and impregnation ratio of 1.5 showed a larger surface area and total pore volume (2229 m2/g and 1.6 cm3/g) than AC produced from rice husk (1941 m2/g and 1.5 cm3/g); no definite and general conclusion can be made about the superiority of rice straw as the raw material. This is attributed to the close characteristics of the products, the large variety of rice crop residues, and the strong influence of the operating conditions on the properties of products. The rice husk– and rice straw–based activated carbons had no significant difference in their functional groups and chemical characteristics. The amorphous silica nanosize particles (< 50 nm) were obtained from both raw materials; however, the presence of little crystalline phase impurities (potassium chloride) and silica (tridymite and coesite) was also observed. The most important superiority of rice husk to rice straw was its larger silica production yield from the initial biochar (40% compared to 25%).





Similar content being viewed by others
References
Rice knowledge bank, By-products (2019) International Rice Research Institute (RRI), IRRI. http://www.knowledgebank.irri.org/step-by-stepproduction/postharvest/rice-by-products. Accessed 5 Sept 2019
Bakker R, Elbersen W, Poppens R, Lesschen J (2013) Rice straw and wheat straw - potential feedstocks for the biobased economy, NL Agency, Ministry of Economic Affairs. https://library.wur.nl/WebQuery/wurpubs/448025. Accessed 20 May 2020
Beidaghy Dizaji H, Zeng T, Hartmann I, Enke D, Schliermann T, Lenz V, Bidabadi M (2019) Generation of high quality biogenic silica by combustion of rice husk and rice straw combined with pre-and post-treatment strategies—a review. Appl Sci 9:1083. https://doi.org/10.3390/app9061083
Moayedi H, Aghel B, Abdullahi MAM, Nguyen H, Rashid ASA (2019) Applications of rice husk ash as green and sustainable biomass. J Clean Prod 237:117851. https://doi.org/10.1016/j.jclepro.2019.117851
Liu D, Zhang W, Huang W (2019) Effect of removing silica in rice husk for the preparation of activated carbon for supercapacitor applications. Chin Chem Lett 30:1315–1319. https://doi.org/10.1016/j.cclet.2019.02.031
Shen Y, Zhang N, Fu Y (2019) Synthesis of high-performance hierarchically porous carbons from rice husk for sorption of phenol in the gas phase. J Environ Manag 241:53–58. https://doi.org/10.1016/j.jenvman.2019.04.012
Liou TH, Wang PY (2020) Utilization of rice husk wastes in synthesis of graphene oxide-based carbonaceous nanocomposites. Waste Manag 108:51–61. https://doi.org/10.1016/j.wasman.2020.04.029
Priya AS, Devi KS (2020) Designing biomass rice husk silica as an efficient catalyst for the synthesis of biofuel additive n-butyl levulinate. Bioenergy Res 13:746–756. https://doi.org/10.1007/s12155-020-10128-5
Sastré-Hernández J, Aguilar-Hernández JR, Santoyo-Salazar J, Alfaro HM, Hoyos-García JE, Tufiño-Velázquez M, Contreras-Puente G (2020) Influence of sodium peroxide during the synthesis of SiO2 obtained from rice husk. Mater Sci Semicond Process 114:105087. https://doi.org/10.1016/j.mssp.2020.105087
Halimah MK, Umar SA, Chan KT, Latif AA, Azlan MN, Abubakar AI, Hamza AM (2019) Study of rice husk silicate effects on the elastic, physical and structural properties of borotellurite glasses. Mater Chem Phys 238:121891. https://doi.org/10.1016/j.matchemphys.2019.121891
Liu Y, Guo Y, Zhu Y, An D, Gao W, Wang Z, Ma Y, Wang Z (2011) A sustainable route for the preparation of activated carbon and silica from rice husk ash. J Hazard Mater 186:1314–1319. https://doi.org/10.1016/j.jhazmat.2010.12.007
Liu Y, Guo Y, Gao W, Wang Z, Ma Y, Wang Z (2012) Simultaneous preparation of silica and activated carbon from rice husk ash. J Clean Prod 32:204–209. https://doi.org/10.1016/j.jclepro.2012.03.021
Schaefer S, Muñiz G, Izquierdo MT, Mathieu S, Ballinas-Casarrubias ML, González-Sánchez G, Celzard A, Fierro V (2017) Rice straw-based activated carbons doped with SiC for enhanced hydrogen adsorption. Int J Hydrog Energy 42:11534–11540. https://doi.org/10.1016/j.ijhydene.2017.02.043
Murge P, Dinda S, Roy S (2018) Adsorbent from rice husk for CO2 capture: synthesis, characterization, and optimization of parameters. Energy Fuel 32:10786–10795. https://doi.org/10.1021/acs.energyfuels.8b02411
Li M, Xiao R (2019) Preparation of a dual pore structure activated carbon from rice husk char as an adsorbent for CO2 capture. Fuel Process Technol 186:35–39. https://doi.org/10.1016/j.fuproc.2018.12.015
Fang F, Zhou J, Yang J (2012) Poly-generation of activated carbon and silica from rice husk charcoal. TCSAE, 28:184–191. https://doi.org/10.3969/j.issn.1002-6819.2012.23.025
Meng MW, Qi CL, Liu QY, Lv L, Ai H, Zhou ZM, Zhang T, Jiang H, Kang CY, Huang SY (2014) Optimization of production conditions for activated carbons from rice husk by potassium carbonate using response surface methodology. Adv Mater Res 1053:303–310. https://doi.org/10.4028/www.scientific.net/AMR.1053.303
Chen JY, Feng XY, Shi ZX (2012) Preparation of mixed-activated carbon from the off-silicon rice husk. J Funct Mater 43:3278–3281
Nguyen TD, Ryu JK, Bramhe Sachin N, Kim TN (2013) Performance of electric double layers capacitor using activated carbon materials from rice husk as electrodes. Korean J Mater Res 23:643–648. https://doi.org/10.3740/MRSK.2013.23.11.643
Liao Q, Li H, Liu Y, Liao L (2015) Optimizing preparation of activated carbons from rice husk by K2CO3 chemical activation. TCSAE 31:256–261. https://doi.org/10.11975/j.issn.1002-6819.2015.11.037
Mai TT, Vu DL, Huynh DC,Wu NL, Le AT (2019) Cost-effective porous carbon materials synthesized by carbonizing rice husk and K2CO3 activation and their application for lithium-sulfur batteries. Journal of Science: Adv Mat & Dev, 4:223–229. https://doi.org/10.1016/j.jsamd.2019.04.009
Mestre AS, Freire C, Pires J, Carvalho AP, Pinto ML (2014) High performance microspherical activated carbons for methane storage and landfill gas or biogas upgrade. J Mater Chem A 2:15337–15344. https://doi.org/10.1039/C4TA03242J
Sevilla M, Fuertes AB (2016) A green approach to high-performance supercapacitor electrodes: the chemical activation of hydrochar with potassium bicarbonate. ChemSusChem 9:1–10. https://doi.org/10.1002/cssc.201600426
Yakout SM, Metwally SS, El-Zakla T (2013) Uranium sorption onto activated carbon prepared from rice straw: competition with humic acids. Appl Surf Sci 280:745–750. https://doi.org/10.1016/j.apsusc.2013.05.055
Mashhadi S, Sohrabi R, Javadian H, Ghasemi M, Tyagi I, Shilpi A, Gupta VK (2016) Rapid removal of Hg (II) from aqueous solution by rice straw activated carbon prepared by microwave-assisted H2SO4 activation: kinetic, isotherm and thermodynamic studies. J Mol Liq 215:144–153. https://doi.org/10.1016/j.molliq.2015.12.040
ASTM D3838-05 (2017) Standard test method for pH of activated, carbon. ASTM International, West Conshohocken. http://www.astm.org. Accessed 08 May 2020
Sharifian S, Sotudeh-Gharebagh R, Zarghami R, Tanguy P, Mostoufi N (2019) Uncertainty in chemical process systems engineering: a critical review. Rev Chem Eng (ahead-of-print) 20180067. https://doi.org/10.1515/revce-2018-0067
Omatola KM, Onojah AD (2009) Elemental analysis of rice husk ash using X – ray fluorescence technique. Int J Phys Sci 4:189–193
Igwebike-Ossi CD (2016) Elemental analysis of rice husk ash using proton-induced X-ray emission (Pixe) spectrometry. Int J Appl Chem 12:233–242
Siddique R, Cachim P (2018) Waste and Supplementary Cementitious Materials in Concrete: Characterisation, Properties and Applications, Woodhead Publishing, UK
Costa JAS, Paranhos CM (2018) Systematic evaluation of amorphous silica production from rice husk ashes. J Clean Prod 192:688–697. https://doi.org/10.1016/j.jclepro.2018.05.028
Rafiee E, Shahebrahimi S, Feyzi M, Shaterzadeh M (2012) Optimization of synthesis and characterization of nanosilica produced from rice husk (a common waste material). Int Nano Lett 2:29. https://doi.org/10.1186/2228-5326-2-29
Lu X, Shih K, Li XY, Liu G, Zeng EY, Wang F (2016) Accuracy and application of quantitative X-ray diffraction on the precipitation of struvite product. Water Res 90:9–14. https://doi.org/10.1016/j.watres.2015.12.014
Sharma G, Arya SK, Singh K (2018) Optical and thermal properties of glasses and glass-ceramics derived from agricultural wastes. Ceram Int 44:947–952. https://doi.org/10.1016/j.ceramint.2017.10.027
Cychosz KA, Guillet-Nicolas R, García-Martínez J, Thommes M (2017) Recent advances in the textural characterization of hierarchically structured nanoporous materials. Chem Soc Rev 46:389–414. https://doi.org/10.1039/C6CS00391E
Tan YH, Davis JA, Fujikawa K, Ganesh NV, Demchenko AV, Stine KJ (2012) Surface area and pore size characteristics of nanoporous gold subjected to thermal, mechanical, or surface modification studied using gas adsorption isotherms, cyclic voltammetry, thermogravimetric analysis, and scanning electron microscopy. J Mater Chem 22:6733–6745. https://doi.org/10.1039/C2JM16633J
Luo Y, Li D, Chen Y, Sun X, Cao Q, Liu X (2019) The performance of phosphoric acid in the preparation of activated carbon-containing phosphorus species from rice husk residue. J Mater Sci 54:5008–5021. https://doi.org/10.1007/s10853-018-03220-x
Puziy AM, Poddubnaya OI, Ziatdinov AM (2006) On the chemical structure of phosphorus compounds in phosphoric acid-activated carbon. Appl Surf Sci 252:8036–8038. https://doi.org/10.1016/j.apsusc.2005.10.044
Yalcin N, Sevinc V (2001) Studies on silica obtained from rice husk. Ceram Int 27:219–224. https://doi.org/10.1016/S0272-8842(00)00068-7
Na LI, Almarri M, ZHA QF (2011) The role of surface oxygen-containing functional groups in liquid-phase adsorptive denitrogenation by activated carbon. New Carbon Mater 26:470–478. https://doi.org/10.1016/S1872-5805(11)60093-0
Marsh H, Reinoso FR (2006) Activated carbon. Elsevier, Burlington
Scala F (2001) Simulation of mercury capture by activated carbon injection in incinerator flue gas. 1. In-duct removal. Environ Sci Technol 35:4367–4372. https://doi.org/10.1021/es010065h
Guillossou R, Le Roux J, Mailler R, Pereira-Derome CS, Varrault G, Bressy A, Vulliet E, Morlay C, Nauleau F, Rocher V, Gasperi J (2020) Influence of dissolved organic matter on the removal of 12 organic micropollutants from wastewater effluent by powdered activated carbon adsorption. Water Res 172:115487. https://doi.org/10.1016/j.watres.2020.115487
Pisharody L, Suresh S, Mukherji S (2020) Evaluation of adsorbents and eluents for application in virus concentration and adsorption-desorption isotherms for coliphages. Chem Eng J 403:126267. https://doi.org/10.1016/j.cej.2020.126267
Kamran U, Park SJ (2020) MnO2-decorated biochar composites of coconut shell and rice husk: an efficient lithium ions adsorption-desorption performance in aqueous media. Chemosphere 260:127500. https://doi.org/10.1016/j.chemosphere.2020.127500
Nguyen HT, Van TN, Thi PN, Lan PDT, Pham HT, Thi HC (2020) Low-cost hydrogel derived from agro-waste for veterinary antibiotic removal: optimization, kinetics, and toxicity evaluation. Environ Technol Innov 20:101098. https://doi.org/10.1016/j.eti.2020.101098
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khoshnood Motlagh, E., Asasian-Kolur, N. & Sharifian, S. A comparative study on rice husk and rice straw as bioresources for production of carbonaceous adsorbent and silica. Biomass Conv. Bioref. 12, 5729–5738 (2022). https://doi.org/10.1007/s13399-020-01145-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01145-7