Abstract
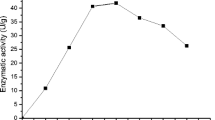
This study investigated for the first time the production of β-glucosidase from Penicillium roqueforti ATCC 10110 by solid-state fermentation using the forage palm (Nopalea cochenillifera) as a substrate. For the optimization of incubation parameters, time, and temperature, the central composite rotational statistical model was used, resulting in an increase in enzymatic activity, by 94.70% with a maximum yield of 935.07 ± 21.70 IU/g at 23 °C and 56% moisture content. The β-glucosidase produced to show higher reactivity and stability at pH 5.0 at 52 °C. In addition to good halotolerance, maintaining 139.93% and 95.34% of the residual activity in the reaction and 57.93% and 94.28% after 24 h of incubation. The addition of Na+, Fe2 +, Mg2+, EDTA, Triton X-100, lactose, and dichloromethane improved β-glucosidase activity, while Ca2+, Zn2+, and Co2+ were not expressed, and SDS, ethanol, acetone, and methanol were inhibitors. Therefore, it was possible to produce β-glucosidase with a halotolerant profile, indicating a promising alternative to obtain this enzyme for biotechnological and industrial applications.


Similar content being viewed by others
References
Gosling SN, Arnell NW (2016) A global assessment of the impact of climate change on water scarcity. Clim Change 134:371–385. https://doi.org/10.1007/s10584-013-0853-x
Alencar BRA, Dutra ED, Sampaio EVDSB, Menezes RSC, Morais JMA (2018) Enzymatic hydrolysis of cactus pear varieties with high solids loading for bioethanol production. Bioresour Technol 250:273–280. https://doi.org/10.1016/j.biortech.2017.11.042
Santos NT, Dutra ED, do Prado AG, Leite FCB, de Souza RDFR, dos Santos DC, Menezes RSC (2016) Potential for biofuels from the biomass of prickly pear cladodes: challenges for bioethanol and biogas production in dry areas. Biomass and Bioenerg 85:215–222. https://doi.org/10.1016/j.biombioe.2015.12.005
De Souza Filho PF, Ribeiro VT, dos Santos ES, de Macedo GR (2016) Simultaneous saccharification and fermentation of cactus pear biomass-evaluation of using different pretreatments. Ind Crop Prod 89:425–433. https://doi.org/10.1016/j.indcrop.2016.05.028
Chiacchio FPB, Mesquita AS, Santos JRD (2006) Palma forrageira: uma oportunidade econômica ainda desperdiçada para o semiárido baiano. Bahia Agrícola 7:39–49
Santos TC, dos Santos RN, Silva TP, Machado FPP, Bonomo RCF, Franco M (2016) Prickly palm cactus husk as a raw material for production of ligninolytic enzymes by Aspergillus niger. Food Sci Biotechnol 25:205–211. https://doi.org/10.1007/s10068-016-0031-9
Santos TC, de Brito AR, Bonomo RCF, Pires AJV, Aguiar-Oliveira E, Silva TP, Franco M (2017) Statistical optimization of culture conditions and characterization for ligninolytic enzymes produced from Rhizopus sp. using prickly palm cactus husk. Chem Eng Commun 204:55–63. https://doi.org/10.1590/1983-21252016v29n126rc
Santos TC, Abreu Filho G, Brito AR, Pires AJV, Bonomo RCF, Franco M (2016) Production and characterization of cellulolytic enzymes by Aspergillus niger and Rhizopus sp. by solid state fermentation of prickly pear. Revista Caatinga 29:222–233. https://doi.org/10.1590/1983-21252016v29n126rc
Viniegra-González G, Favela-Torres E, Aguilar CN, de Jesus R-GS, Dıaz-Godınez G, Augur C (2003) Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem Eng J 13:157–167. https://doi.org/10.1016/S1369-703X(02)00128-6
Watanabe A, Suzuki M, Ujiie S, Gomi K (2016) Purification and enzymatic characterization of a novel β-1,6-glucosidase from Aspergillus oryzae. J Biosci Bioeng 121:259–264. https://doi.org/10.1016/j.jbiosc.2015.07.011
Pandey A, Francis F, Sabu A, Nampoothiri KM, Ramachandran S, Ghosh S, Szakacs G (2003) Use of response surface methodology for optimizing process parameters for the production of α-amylase by Aspergillus oryzae. Biochem Eng J 15:107–115. https://doi.org/10.1016/S1369-703X(02)00192-4
Ferraz JLAA, Souza LO, Soares GA, Coutinho JP, de Oliveira JR, Aguiar-Oliveira E, Franco M (2018) Enzymatic saccharification of lignocellulosic residues using cellulolytic enzyme extract produced by Penicillium roqueforti ATCC 10110 cultivated on residue of yellow mombin fruit. Bioresour Technol 248:214–220. https://doi.org/10.1016/j.biortech.2017.06.048
Marques GL, dos Santos RN, Silva TP, Ferreira MLO, Aguiar-Oliveira E, de Oliveira JR, Franco M (2017) Production and characterisation of xylanase and endoglucanases produced by Penicillium roqueforti ATCC 10110 through the solid-state fermentation of rice husk residue. Waste Biomass Valori 9:2061–2069. https://doi.org/10.1007/s12649-017-9994-x
Meleiro LP, Carli S, Fonseca-Maldonado R, da Silva TM, Zimbardi ALRL, Ward RJ, Furriel RPM (2018) Over expression of a cellobiose-glucose-halotolerant endoglucanase from Scytalidium thermophilum. Appl Biochem Biotech 185:316–333. https://doi.org/10.1007/s12010-017-2660-8
Sohail M, Siddiqi R, Ahmad A, Khan SA (2009) Cellulase production from Aspergillus niger MS82: effect of temperature and pH. New Biotechnol 25:437–441. https://doi.org/10.1016/j.nbt.2009.02.002
Souza LO, de Brito AR, Bonomo RCF, Santana NB, Ferraz JLAA, Aguiar-Oliveira E, Franco M (2018) Comparison of the biochemical properties between the xylanases of Thermomyces lanuginosus (Sigma®) and excreted by Penicillium roqueforti ATCC 10110 during the solid state fermentation of sugarcane bagasse. Biocatal Agric Biotechnol 16:277–284. https://doi.org/10.1016/j.bcab.2018.08.016
Xu J, He B, Wu B, Wang B, Wang C, Hu L (2014) An ionic liquid tolerant cellulase derived from chemically polluted microhabitats and its application in in situ saccharification of rice straw. Bioresour Technol 157:166–173. https://doi.org/10.1016/j.biortech.2014.01.10
Prasanna HN, Ramanjaneyulu G, Rajasekhar Reddy B (2016) Optimization of cellulase production by Penicillium sp. Biotech 6:162. https://doi.org/10.1007/s13205-016-0483-x
Meleiro LP, Carli S, Fonseca-Maldonado R, da Silva TM, Zimbardi ALRL, Ward RJ, Furriel RPM (2018) Over expression of a cellobiose-glucose-halotolerant endoglucanase from Scytalidium thermophilum. Appl Biochem Biotech 185:316–333. https://doi.org/10.1007/s12010-017-2660-8
Matsuura M, Sasaki J, Murao S (1995) Studies on β-glucosidases from soybeans that hydrolyze daidzin and genistin: isolation and characterization of an isozyme. Biosci Biotech Bioch 59:1623–1627. https://doi.org/10.1271/bbb.59.1623
Pereira CJ, Giese EC, de Souza Moretti MM, dos Santos Gomes AC, Perrone OM, Boscolo M, Martins DAB (2017) Effect of metal ions, chemical agents and organic compounds on lignocellulolytic enzymes activities. In Enzyme inhibitors and activators IntechOpen 6:139–164. https://doi.org/10.5772/65934
Aliyah A, Alamsyah G, Ramadhani R, Hermansyah H (2017) Production of α-amylase and β-glucosidase from Aspergillus niger by solid state fermentation method on biomass waste substrates from rice husk, bagasse and corn cob. Energy Procedia 136:418–423. https://doi.org/10.1016/j.egypro.2017.10.269
Salim AA, Grbavčić S, Šekuljica N, Stefanović A, Tanasković SJ, Luković N, Knežević-Jugović Z (2017) Production of enzymes by a newly isolated Bacillus sp. TMF-1 in solid state fermentation on agricultural by-products: the evaluation of substrate pretreatment methods. Bioresour Technol 228:193–200. https://doi.org/10.1016/j.biortech.2016.12.081
Ohara A, Santos JGD, Angelotti JAF, Barbosa PPM, Dias FFG, Bagagli MP, Castro RJSD (2018) A multicomponent system based on a blend of agroindustrial wastes for the simultaneous production of industrially applicable enzymes by solid-state fermentation. Food Sci Technol (AHEAD) 38:131–137. https://doi.org/10.1590/1678-457x.17017
Park AR, Hong JH, Kim JJ, Yoon JJ (2012) Biochemical characterization of an extracellular β-glucosidase from the fungus, Penicillium italicum, isolated from rotten citrus peel. Mycobiology 40:173–180. https://doi.org/10.5941/MYCO.2012.40.3.173
Jeya M, Joo AR, Lee KM, Tiwari MK, Lee KM, Kim SH, Lee JK (2010) Characterization of β-glucosidase from a strain of Penicillium purpurogenum KJS506. Appl Microbiol Biotechnol 86:1473–1484. https://doi.org/10.1007/s00253-009-2395-8
Nehad EA, Yoness MF, Reem AA (2019) Optimization and purification of cellulase produced by Penicillium decumbens and its application. Egypt Pharmaceut J 18:391. https://doi.org/10.4103/epj.epj_31_19
Ng IS, Li CW, Chan SP, Chir JL, Chen P, Tong CG, Yu SM, Ho TH (2010) High-level production of a thermoacidophilic β-glucosidase from Penicillium citrinum YS40-5 by solid-state fermentation with rice bran. Bioresour. Technol 101:1310–1317. https://doi.org/10.1016/j.biortech.2009.08.049
Miyano H, Toyo’oka T, Imai K, Nakajima T (1985) Influences of metal ions on the reaction of amino and imino acids with fluorogenic reagents. Anal Biochem 150:125–130. https://doi.org/10.1016/0003-2697(85)90450-6
De Oliveira RP, dos Santos BV, Costa L, Henrique MA, Pasquini D, Baffi MA (2017) Xylanase and β-glucosidase production by Aspergillus fumigatus using commercial and lignocellulosic substrates submitted to chemical pre-treatments. Ind Crop Prod 95:453–459. https://doi.org/10.1016/j.indcrop.2016.10.055
Nishida VS, de Oliveira RF, Brugnari T, Correa RCG, Peralta RA, Castoldi R, Peralta RM (2018) Immobilization of Aspergillus awamori β-glucosidase on commercial gelatin: an inexpensive and efficient process. Int J Biol Macromol 111:1206–1213. https://doi.org/10.1016/j.ijbiomac.2018.01.146
Méndez Arias J, Modesto LFA, Polikarpov I, Pereira JN (2016) Design of an enzyme cocktail consisting of different fungal platforms for efficient hydrolysis of sugarcane bagasse: optimization and synergism studies. Biotechnol prog 32:1222–1229. https://doi.org/10.1002/btpr.2306
Zhang H, Sang Q (2012) Statistical optimization of cellulases production by Penicillium chrysogenum QML-2 under solid-state fermentation and primary application to chitosan hydrolysis. World J Microb Biot 28:1163–1174. https://doi.org/10.1007/s11274-011-0919-8
Santos FR, Garcia NFL, da Paz MF, Fonseca GG, Leite RSR (2016) Production and characterization of β-glucosidase from Gongronella butleri by solid-state fermentation. Afr J Biotechnol 15:633–641. https://doi.org/10.5897/AJB2015.15025
Morais TPD, Barbosa PMG, Garcia NFL, Rosa-Garzon NGD, Fonseca GG, Paz MFD, Leite RSR (2018) Catalytic and thermodynamic properties of β-glucosidases produced by Lichtheimia corymbifera and Byssochlamys spectabilis. Prep Biochem Biotech 48:777–786. https://doi.org/10.1080/10826068.2018.1509083
Ariff INM, Bahrin EK, Ramli N, Abd-Aziz S (2017) Direct use of spent mushroom substrate from Pleurotus pulmonarius as a readily delignified feedstock for cellulase production. Waste Biomass Valori 10:839–850. https://doi.org/10.1007/s12649-017-0106-8
Shin KC, Seo MJ, Kim DW, Yeom SJ, Kim YS (2019) Characterization of β-glycosidase from Caldicellulosiruptor owensensis and its application in the production of platycodin D from balloon flower leaf. Catalysts 9:1025. https://doi.org/10.3390/catal9121025
Kuo HP, Wang R, Huang CY, Lai JT, Lo YC, Huang ST (2018) Characterization of an extracellular β-glucosidase from Dekkera bruxellensis for resveratrol production. J Food Drug Anal 26:163–171. https://doi.org/10.1016/j.jfda.2016.12.016
Alarid-García C, Escamilla-Silva EM (2017) Comparative study of the production of extracellular β-glucosidase by four different strains of Aspergillus using submerged fermentation. Prep Biochem Biotech 47:597–610. https://doi.org/10.1080/10826068.2017.1286598
Ahmed A, Aslam M, Ashraf M, Nasim F, Ul-Hassan Batool K, Bibi A (2017) Microbial β-glucosidases: screening, characterization, cloning and applications. J App Environ Microbiol 5:57–73. https://doi.org/10.12691/jaem-5-2-2
Fusco FA, Fiorentino G, Pedone E, Contursi P, Bartolucci S, Limauro D (2018) Biochemical characterization of a novel thermostable β-glucosidase from Dictyoglomus turgidum. Int J Biol Macromol 113:783–791. https://doi.org/10.1016/j.ijbiomac.2018.03.018
Oh JM, Lee JP, Baek SC, Kim SG, Do J, Kim YJ, Kim H (2018) Characterization of two extracellular β-glucosidases produced from the cellulolytic fungus Aspergillus sp. YDJ216 and their potential applications for the hydrolysis of flavone glycosides. Int J Biol Macromol 111:595–603. https://doi.org/10.1016/j.ijbiomac.2018.01.020
Li Y, Hu X, Sang J, Zhang Y, Zhang H, Lu F, Liu F (2018) An acid-stable β-glucosidase from Aspergillus aculeatus: gene expression, biochemical characterization and molecular dynamics simulation. Int J Biol Macromol 119:462–469. https://doi.org/10.1016/j.ijbiomac.2018.07.165
Asha P, Divya J, Singh IB (2016) Purification and characterisation of processive-type endoglucanase and β-glucosidase from Aspergillus ochraceus MTCC 1810 through saccharification of delignified coir pith to glucose. Bioresour Technol 213:245–248. https://doi.org/10.1016/j.biortech.2016.03.013
Dong W, Xue M, Zhang Y, Xin F, Wei C, Zhang W, Jiang M (2017) Characterization of a β-glucosidase from Paenibacillus species and its application for succinic acid production from sugarcane bagasse hydrolysate. Bioresour Technol 241:309–316. https://doi.org/10.1016/j.biortech.2017.05.141
Ahmed SS, Akhter M, Sajjad M, Gul R, Khurshid S (2019) Soluble production, characterization, and structural aesthetics of an industrially important thermostable β-glucosidase from Clostridium thermocellum in Escherichia coli. BioMed Research International 8:2019–2018. https://doi.org/10.1155/2019/9308593
Ishida N, Okubo A, Kawai H, Yamazaki S, Toda S (1980) Interaction of amino acids with transition metal ions in solution (I) solution structure of L-lysine with Co (II) and Cu (II) ions as studied by nuclear magnetic resonance spectroscopy. Agric Biol Chem 44:263–270. https://doi.org/10.1080/00021369.1980.10863934
Mandels M, Reese ET (1965) Inhibition of cellulases. Annu Rev Phytopathol 3:85–102. https://doi.org/10.1146/annurev.py.03.090165.000505
Ovalle S, Cavello I, Brena BM, Cavalitto S, González-Pombo P (2018) Production and characterization of a β-glucosidase from Issatchenkia terricola and its use for hydrolysis of aromatic precursors in Cabernet Sauvignon wine. LWT 87:515–522. https://doi.org/10.1016/j.lwt.2017.09.026
Wu J, Geng A, Xie R, Wang H, Sun J (2018) Characterization of cold adapted and ethanol tolerant β-glucosidase from Bacillus cellulosilyticus and its application for directed hydrolysis of cellobiose to ethanol. Int J Biol Macromol 109:872–879. https://doi.org/10.1016/j.ijbiomac.2017.11.072
Martins EDS, Gomes E, da Silva R, Junior RB (2019) Production of cellulases by Thermomucor indicae-seudaticae: characterization of a thermophilic β-glucosidase. Prep Biochem Biotech 49:830–836. https://doi.org/10.1080/10826068.2019.1625060
Onat S, Savaş E (2019) Immobilization and characterization of β-glucosidase from gemlik olive (Olea europea l.) responsible for hydrolization of oleuropein. Ital J Food Sci 31:1120–1770. https://doi.org/10.14674/IJFS-1529
Hsieh CWC, Cannella D, Jørgensen H, Felby C, Thygesen LG (2014) Cellulase inhibition by high concentrations of monosaccharides. J Agric Food Chem 62:3800–3805. https://doi.org/10.1021/jf5012962
Kristensen JB, Börjesson J, Bruun MH, Tjerneld F, Jørgensen H (2007) Use of surface active additives in enzymatic hydrolysis of wheat straw lignocellulose. Enzyme Microb Technol 40:888–895. https://doi.org/10.1016/j.enzmictec.2006.07.014
Dhillon GS, Kaur S, Brar SK, Verma M (2012) Potential of apple pomace as a solid substrate for fungal cellulase and hemicellulase bioproduction through solid-state fermentation. Ind Crop Prod 38:6–13. https://doi.org/10.1016/j.indcrop.2011.12.036
Sun H, Ge X, Hao Z, Peng M (2010) Cellulase production by Trichoderma sp. on apple pomace under solid state fermentation. Afr J Biotechnol 9:163–166
Hwang EJ, Lee YS, Choi YL (2018) Cloning, purification, and characterization of the organic solvent tolerant β-glucosidase, OaBGL84, from Olleya aquimaris DAU311. App Biol Chem 61:325–336. https://doi.org/10.1007/s13765-018-0361-9
Batra J, Mishra S (2013) Organic solvent tolerance and thermostability of a β-glucosidase co-engineered by random mutagenesis. J Mol Catal B Enzym 96:61–66. https://doi.org/10.1016/j.molcatb.2013.07.002
Pogorevc M, Stecher H, Faber K (2002) A caveat for the use of log P values for the assessment of the biocompatibility of organic solvents. Biotechnol Lett 24:857–860. https://doi.org/10.1023/A:1015598523282
Laane C, Boeren S, Vos K, Veeger C (1987) Rules for optimization of biocatalysis in organic solvents. Biotechnol Bioeng 30:81–87. https://doi.org/10.1002/bit.260300112
Pasin TM, Salgado JCS, Scarcella ASA, de Oliveira TB, de Lucas RC, Cereia M, Polizeli MLTM (2020) A halotolerant endo-1,4-β-xylanase from Aspergillus clavatus with potential application for agroindustrial residues saccharification. Appl Biochem Biotech 191:1–16. https://doi.org/10.1007/s12010-020-03232-x
Silva TP, Fabiana S, dos Santos CWV, Franco M, Caetano LC, Pereira HJV (2018) Production, purification, characterization and application of a new halotolerant and thermostable endoglucanase of Botrytis ricini URM 5627. Bioresour Technol 270:263–269. https://doi.org/10.1016/j.biortech.2018.09.022
Park MS, Oh SY, Fong JJ, Houbraken J, Lim YW (2019) The diversity and ecological roles of Penicillium in intertidal zones. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-49966-5
Madern D, Ebel C, Zaccai G (2000) Halophilic adaptation of enzymes. Extremophiles 4:91–98. https://doi.org/10.1007/s007920050142
Delgado-García M, Valdivia-Urdiales B, Aguilar-González CN, Contreras-Esquivel JC, Rodríguez-Herrera R (2012) Halophilic hydrolases as a new tool for the biotechnological industries. J Sci Food Agric 92:2575–2580. https://doi.org/10.1002/jsfa.5860
Passos FD, Pereira JN, de Castro AM (2018) A comparative review of recent advances in cellulases production by Aspergillus, Penicillium and Trichoderma strains and their use for lignocellulose deconstruction. Current Opinion in Green and Sustainable Chemistry 14:60–66. https://doi.org/10.1016/j.cogsc.2018.06.003
Salgado JCS, Meleiro LP, Carli S, Ward RJ (2018) Glucose tolerant and glucose stimulated β-glucosidases–a review. Bioresour Technol 267:704–713. https://doi.org/10.1016/j.biortech.2018.07.137
Santos SR, Nayara FLG, Marcelo FP, Gustavo GF, Rodrigo S, Otilde ESRL (2016) Production and characterization of -glucosidase from Gongronella butleri by solid-state fermentation. Afr J Biotechnol 15:633–641. https://doi.org/10.5897/ajb2015.15025
Acknowledgments
The authors would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for their financial support and the State University of Santa Cruz (UESC) for its administrative and technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
das Neves, C.A., de Menezes, L.H.S., Soares, G.A. et al. Production and biochemical characterization of halotolerant β-glucosidase by Penicillium roqueforti ATCC 10110 grown in forage palm under solid-state fermentation. Biomass Conv. Bioref. 12, 3133–3144 (2022). https://doi.org/10.1007/s13399-020-00930-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00930-8