Abstract
This paper reports the results of a thermodynamic analysis conducted for ethanol dehydrogenation. In order to be implemented, the computational code required the choice of a representative set of species, which was selected from performance data of Cu-based catalysts conducted with different residence times (W/F) and supports. Although a major by-product, methyl ethyl ketone (MEK) was removed from the assembly, because it has demonstrated to be thermodynamically more stable than the other organic compounds, which led ethanol to be converted to MEK only, the obtained results were quite representative. The two major organic products (ethyl acetate and acetaldehyde) competed with each other, indicating that ethanol is converted to one of the two substances at the expense of the other. Regardless of the residence time or the type of support employed, the catalytic conversions (obtained from the literature) always remained below the thermodynamic threshold, indicating that thermodynamics is essential to foresee catalyst limitations: maximum ethanol conversion and selectivity to ethyl acetate.

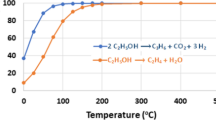
Gibbs energies of the various reactions involved in the process of ethanol dehydrogenation











Similar content being viewed by others
References
Annen SP, Bambagioni V, Bevilacqua M, Filippi J, Marchionni A, Oberhauser W, Schönberg H, Vizza F, Bianchini C, Grützmacher H (2010) A biologically inspired organometallic fuel cell (OMFC) that converts renewable alcohols into energy and chemicals. Angew Chem Int Ed 49(40):7229–7233
Dutia P (2004) Ethyl acetate: a techno-commercial profile. Chemical Weekly 10:179–186
Ogata Y, Kawasaki A (1969) Alkoxide transfer from aluminium alkoxide to aldehyde in the Tishchenko reaction. Tetrahedron 25(4):929–935
Rothenberg G (2008) Catalysis—concepts and green applications. Wiley-VCH, Weinheim
Colley SW, Fawcett CR, Rathmell C, Tuck MWM (2004) Process for the preparation of ethyl acetate. US Patent 6,809,217, Davy Process Technology Limited
Inui K, Kurabayashi T, Sato S (2002) Direct synthesis of ethyl acetate from ethanol carried out under pressure. J Catal 212(2):207–215
Colley SW, Tabatabaei J, Waugh KC, Wood MA (2005) The detailed kinetics and mechanism of ethyl ethanoate synthesis over a Cu/Cr2O3 catalyst. J Catal 236(1):21–33
Sánchez AB, Homs N, Fierro JLG, de la Piscina PR (2005) New supported Pd catalysts for the direct transformation of ethanol to ethyl acetate under medium pressure conditions. Catal Today 107–108:431–435
Natal-Santiago MA, Hill JM, Dumesic JA (1999) Studies of the adsorption of acetaldehyde, methyl acetate, ethyl acetate, and methyl trifluoroacetate on silica. J Mol Catal A Chem 140(2):199–214
Santiago MAN, Sánchez-Castillo MA, Cortright RD, Dumesic JA (2000) Catalytic reduction of acetic acid, methyl acetate, and ethyl acetate over silica-supported copper. J Catal 193(1):16–28
Inui K, Kurabayashi T, Sato S (2002) Direct synthesis of ethyl acetate from ethanol over Cu-Zn-Zr-Al-O catalyst. Appl Catal A 237(1–2):53–61
Inui K, Kurabayashi T, Sato S, Ichikawa N (2004) Effective formation of ethyl acetate from ethanol over Cu-Zn-Zr-Al-O catalyst. J Mol Catal A Chem 216(1):147–156
Gaspar AB, Esteves AML, Mendes FMT, Barbosa FG, Appel LG (2009) Chemicals from ethanol—the ethyl acetate one-pot synthesis. Appl Catal A 363(1–2):109–114
Gaspar AB, Barbosa FG, Letichevsky S, Appel LG (2010) The one-pot ethyl acetate syntheses: the role of the support in the oxidative and the dehydrogenative routes. Appl Catal A 380(1–2):113–117
Zonetti PC, Celnik J, Letichevsky S, Gaspar AB, Appel LG (2011) Chemicals from ethanol—the dehydrogenative route of the ethyl acetate one-pot synthesis. J Mol Catal A Chem 334(1–2):29–34
Sato AG, Volanti DP, Meira DM, Damyanova S, Longo E, Bueno JMC (2013) Effect of the ZrO2 phase on the structure and behavior of supported Cu catalysts for ethanol conversion. J Catal 307:1–17
Prasad J, Menon PG (1972) The bifunctional nature of Cu-Al2O3 catalysts in the conversion of ethanol to ethyl acetate. J Catal 26(3):477–481
Manríquez ME, López T, Gómez R, Navarrete J (2004) Preparation of TiO2-ZrO2 mixed oxides with controlled acid-basic properties. J Mol Catal A Chem 220(2):229–237
Zhang L, Pham TN, Faria J, Resasco DE (2015) Improving the selectivity to C4 products in the aldol condensation of acetaldehyde in ethanol over faujasite zeolites. Appl Catal A 504:119–129
Wang Q, Yan X-H, Chen G-H, Han S-J (2003) Measurement and prediction of quaternary azeotropes for cyclohexane + 2-propanol + ethyl acetate + butanone system at elevated pressures. J Chem Eng Data 48(1):66–70
Gursahani KI, Alcalá R, Cortright RD, Dumesic JA (2001) Reaction kinetics measurements and analysis of reaction pathways for conversions of acetic acid, ethanol, and ethyl acetate over silica-supported Pt. Appl Catal A 222(1–2):369–392
Ávila-Neto CN, Dantas SC, Silva FA, Franco TV, Romanielo LL, Hori CE, Assis AJ (2009) Hydrogen production from methane reforming: thermodynamic assessment and autothermal reactor design. J Nat Gas Sci Eng 1(6):205–215
de Ávila CN, Hori CE, de Assis AJ (2011) Thermodynamic assessment of hydrogen production and cobalt oxidation susceptibility under ethanol reforming conditions. Energy 36(7):4385–4395
da Silva AL, Malfatti CF, Müller IL (2009) Thermodynamic analysis of ethanol steam reforming using Gibbs energy minimization method: a detailed study of the conditions of carbon deposition. Int J Hydrogen Energy 34(10):4321–4330
Freitas IC, Damyanova S, Oliveira DC, Marques CMP, Bueno JMC (2014) Effect of Cu content on the surface and catalytic properties of Cu/ZrO2 catalyst for ethanol dehydrogenation. J Mol Catal A Chem 381:26–37
Acknowledgments
The authors would like to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant number 470711/2013-2) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Table S1. Effect of temperature on the number of moles in equilibrium state as a function of temperature (P = 1 bar; P 0 EtOH = 0.374 bar). Data were obtained by both the Lagrange multipliers (LM) and the evaluation of equilibrium constants (EEC) method. Table S2. Effect of temperature on equilibrium ethanol conversion and selectivities as a function of temperature (P = 1 bar; P 0 EtOH = 0.374 bar). Data were obtained by both the Lagrange multipliers (LM) and the evaluation of equilibrium constants (EEC) method. (DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Andrade, R.L., Hori, C.E., Sato, A.G. et al. Thermodynamic assessment of ethyl acetate production via ethanol dehydrogenation. Biomass Conv. Bioref. 7, 59–67 (2017). https://doi.org/10.1007/s13399-016-0213-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-016-0213-y