Abstract
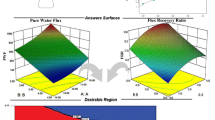
The biodegradability of polycaprolactone (PCL) and the anti-fouling property of TiO2 are utilized for the preparation of membranes for water treatment. Polyethylene glycol (PEG) is added to the polymeric solution to increase the pore formation in the membranes. The composition of PEG and TiO2 nanoparticles was optimized using the central composite design and response surface methodology. Quadratic models were developed for the responses porosity and contact angle, and the optimum compositions obtained for PEG and TiO2 were 6.24 and 1 wt%, respectively. The competency of the membrane for water treatment is studied using different characterization techniques such as TG analysis, FTIR to determine the chemical interactions, UTM to examine the mechanical strength, AFM for membrane roughness determination and morphological analysis using FESEM. A twofold increase in the overall porosity and a twentyfold reduction (approx.) in pore size were observed for the composite membrane from FESEM images. The anti-fouling ability of TiO2 resulted in a flux recovery of about 90% for the composite membrane compared to the PCL–PEG membrane after bovine serum albumin filtration. Also, the presence of TiO2 increased the reversible fouling component, which can be easily removed by cleaning/backwashing. Thus, the PCL–TiO2 combination would be a better alternative for the membranes used in water treatment applications.











Similar content being viewed by others
References
Abedalwafa, M.; Wang, F.; Wang, L.; Li, C.: Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: a review. Rev. Adv. Mater. Sci. 34, 123–140 (2013)
Loh, X.J.: The effect of pH on the hydrolytic degradation of poly(ε-caprolactone)-block-poly(ethylene glycol) copolymers. J. Appl. Polym. Sci. 127, 2046–2056 (2013). https://doi.org/10.1002/app.37712
Heimowska, A.; Morawska, M.; Bocho-Janiszewska, A.: Biodegradation of poly(ε-caprolactone) in natural water environments. Pol. J. Chem. Technol. 19, 120–126 (2017). https://doi.org/10.1515/pjct-2017-0017
Cooper, A.; Oldinski, R.; Ma, H.; Bryers, J.D.: Chitosan-based nanofibrous membranes for antibacterial filter applications. Carbohydr. Polym. 92, 254–259 (2013). https://doi.org/10.1038/jid.2014.371
Rusli, M.S.I.C.; Hassan, M.I.; Sultana, N.; Ismail, A.F.: Characterization of Pcl/zeolite electrospun membrane for the removal of silver in drinking water. J. Technol. 2, 89–95 (2017)
Reshmi, C.R.; Sundaran, S.P.; Juraij, A.; Athiyanathil, S.: Fabrication of superhydrophobic polycaprolactone/beeswax electrospun membranes for high-efficiency oil/water separation. RSC Adv. 7, 2092–2102 (2017). https://doi.org/10.1039/c6ra26123j
Benhacine, F.; Abdellaoui, N.; Arous, O.; Hadj-Hamou, A.S.: Behaviours of poly(ε-caprolactone)/silver-montmorillonite nanocomposite in membrane ultrafiltration for wastewater treatment. Environ. Technol. (United Kingdom) (2018). https://doi.org/10.1080/09593330.2018.1555283
Nivedita, S.; Joseph, S.: Effect of unmodified and modified montmorillonite on the properties of PCL based ultrafiltration membrane for water treatment applications. J. Water Process Eng. 21, 61–68 (2018). https://doi.org/10.1016/j.jwpe.2017.12.002
Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Silveira Villar, L.; Elia Escaleira, L.A.: RSM as a tool for optimization in analytical chemistry. Talanta 76, 965–977 (2008). https://doi.org/10.1016/j.talanta.2008.05.019
Razali, N.F.; Mohammad, A.W.; Hilal, N.; Leo, C.P.; Alam, J.: Optimisation of polyethersulfone/polyaniline blended membranes using response surface methodology approach. Desalination 311, 182–191 (2013). https://doi.org/10.1016/j.desal.2012.11.033
Yiew, T.; Ling, E.; Fadhil, M.; Rezania, S.; Aminudin, E.; Chelliapan, S.; Abdul, A.; Park, J.: Optimization of aluminium recovery from water treatment sludge using response surface methodology. J. Environ. Manag. 228, 13–19 (2018). https://doi.org/10.1016/j.jenvman.2018.09.008
Sadeghian, M.; Sadeghi, M.; Hesampour, M.; Moheb, A.: Application of response surface methodology (RSM) to optimize operating conditions during ultrafiltration of oil-in-water emulsion. Desalin. Water Treat. (2014). https://doi.org/10.1080/19443994.2014.919607
Xu, Z.; Wu, T.; Shi, J.; Teng, K.; Wang, W.; Ma, M.; Li, J.; Qian, X.; Li, C.; Fan, J.: Photocatalytic antifouling PVDF ultrafiltration membranes based on synergy of graphene oxide and TiO2 for water treatment. J. Membr. Sci. 520, 281–293 (2016). https://doi.org/10.1016/j.memsci.2016.07.060
Escobar, I.C.; Van Der Bruggen, B.: Microfiltration and ultrafiltration membrane science and technology. J. Appl. Polym. Sci. (2015). https://doi.org/10.1002/app.42002
Vinodhini, P.A.; Sangeetha, K.; Thandapani, G.; Sudha, P.N.; Jayachandran, V.; Sukumaran, A.: FTIR, XRD and DSC studies of nanochitosan, cellulose acetate and polyethylene glycol blend ultrafiltration membranes. Int. J. Biol. Macromol. 104, 1721–1729 (2017). https://doi.org/10.1016/j.ijbiomac.2017.03.122
Idris, A.; Mat Zain, N.; Noordin, M.Y.: Synthesis, characterization and performance of asymmetric polyethersulfone (PES) ultrafiltration membranes with polyethylene glycol of different molecular weights as additives. Desalination 207, 324–339 (2007). https://doi.org/10.1016/j.desal.2006.08.008
Ma, Y.; Shi, F.; Ma, J.; Wu, M.; Zhang, J.; Gao, C.: Effect of PEG additive on the morphology and performance of polysulfone ultrafiltration membranes. Desalination 272, 51–58 (2011). https://doi.org/10.1016/j.desal.2010.12.054
Liu, Y.; Koops, G.H.; Strathmann, H.: Characterization of morphology controlled polyethersulfone hollow fiber membranes by the addition of polyethylene glycol to the dope and bore liquid solution. J. Membr. Sci. 223, 187–199 (2003). https://doi.org/10.1016/S0376-7388(03)00322-3
Chen, X.; Tang, B.; Luo, J.; Wan, Y.: Towards high-performance polysulfone membrane: the role of PSF-b-PEG copolymer additive. Microporous Mesoporous Mater. 241, 355–365 (2017). https://doi.org/10.1016/j.micromeso.2016.12.032
Kim, I.C.; Lee, K.H.: Effect of poly(ethylene glycol) 200 on the formation of a polyetherimide asymmetric membrane and its performance in aqueous solvent mixture permeation. J. Membr. Sci. 230, 183–188 (2004). https://doi.org/10.1016/j.memsci.2003.11.002
Rekik, S.B.; Gassara, S.; Bouaziz, J.; Deratani, A.; Baklouti, S.: Enhancing hydrophilicity and permeation flux of chitosan/kaolin composite membranes by using polyethylene glycol as porogen. Appl. Clay Sci. 168, 312–323 (2019). https://doi.org/10.1016/j.clay.2018.11.029
Zulkali, M.M.D.; Ahmad, A.L.; Derek, C.J.C.: Membrane application in proteomic studies: preliminary studies on the effect of pH, ionic strength and pressure on protein fractionation. Desalination 179, 381–390 (2005). https://doi.org/10.1016/j.desal.2004.11.084
Behboudi, A.; Jafarzadeh, Y.; Yegani, R.: Preparation and characterization of TiO2 embedded PVC ultrafiltration membranes. Chem. Eng. Res. Des. 114, 96–107 (2016). https://doi.org/10.1016/j.cherd.2016.07.027
Ye, W.; Liu, H.; Lin, F.; Lin, J.; Zhao, S.; Yang, S.; Hou, J.; Zhou, S.; Van Der Bruggen, B.: High-flux nanofiltration membranes tailored by bio-inspired co-deposition of hydrophilic g-C3N4 nanosheets for enhanced selectivity towards organics and salts. Environ. Sci. Nano 6, 2958–2967 (2019). https://doi.org/10.1039/c9en00692c
Lin, J.; Zhang, R.; Ye, W.; Jullok, N.; Sotto, A.; Van der Bruggen, B.: Nano-WS2 embedded PES membrane with improved fouling and permselectivity. J. Colloid Interface Sci. 396, 120–128 (2013). https://doi.org/10.1016/j.jcis.2013.01.028
Lin, J.; Ye, W.; Zhong, K.; Shen, J.; Jullok, N.; Sotto, A.; Van der Bruggen, B.: Enhancement of polyethersulfone (PES) membrane doped by monodisperse Stöber silica for water treatment. Chem. Eng. Process. Process Intensif. 107, 194–205 (2016). https://doi.org/10.1016/j.cep.2015.03.011
Banerjee, A.; Ray, S.K.: PVA modified filled copolymer membranes for pervaporative dehydration of acetic acid-systematic optimization of synthesis and process parameters with response surface methodology. J. Membr. Sci. 549, 84–100 (2018). https://doi.org/10.1016/j.memsci.2017.11.056
Gadekar, M.R.; Ahammed, M.M.: Modelling dye removal by adsorption onto water treatment residuals using combined response surface methodology-artificial neural network approach. J. Environ. Manag. 231, 241–248 (2019). https://doi.org/10.1016/j.jenvman.2018.10.017
Ahmadi, M.; Masoumi, S.; Hassanajili, S.; Esmaeilzadeh, F.: Modification of PES/PU membrane by supercritical CO2 to enhance CO2/CH4 selectivity: fabrication and correlation approach using RSM. Chin. J. Chem. Eng. 26, 2503–2515 (2018). https://doi.org/10.1016/j.cjche.2017.11.018
Wongchitphimon, S.; Wang, R.; Jiraratananon, R.; Shi, L.; Loh, C.H.: Effect of polyethylene glycol (PEG) as an additive on the fabrication of polyvinylidene fluoride-co-hexafluropropylene (PVDF-HFP) asymmetric microporous hollow fiber membranes. J. Membr. Sci. 369, 329–338 (2011). https://doi.org/10.1016/j.memsci.2010.12.008
Emin, C.; Kurnia, E.; Katalia, I.; Ulbricht, M.: Polyarylsulfone-based blend ultrafiltration membranes with combined size and charge selectivity for protein separation. Sep. Purif. Technol. 193, 127–138 (2018). https://doi.org/10.1016/j.seppur.2017.11.008
Kumar, R.; Isloor, A.M.; Ismail, A.F.; Rashid, S.A.; Ahmed, A.: Al: permeation, Antifouling and desalination performance of TiO2 nanotube incorporated PSf/CS blend membranes. Desalination 316, 76–84 (2013). https://doi.org/10.1016/j.desal.2013.01.032
Yang, Y.; Zhang, H.; Wang, P.; Zheng, Q.; Li, J.: The influence of nano-sized TiO2 fillers on the morphologies and properties of PSF UF membrane. J. Membr. Sci. 288, 231–238 (2007). https://doi.org/10.1016/j.memsci.2006.11.019
Yuliwati, E.; Ismail, A.F.: Effect of additives concentration on the surface properties and performance of PVDF ultrafiltration membranes for refinery produced wastewater treatment. Desalination 273, 226–234 (2011). https://doi.org/10.1016/j.desal.2010.11.023
Rajaeian, B.; Heitz, A.; Tade, M.O.; Liu, S.: Improved separation and antifouling performance of PVA thin film nanocomposite membranes incorporated with carboxylated TiO2 nanoparticles. J. Membr. Sci. 485, 48–59 (2015). https://doi.org/10.1016/j.memsci.2015.03.009
Sun, X.; Han, W.; Li, J.; Pang, R.; Fang, X.; Wang, L.; Shen, J.; Li, X.: Self-assembly of TiO2 nanoparticles around the pores of PES ultrafiltration membrane for mitigating organic fouling. J. Membr. Sci. 467, 226–235 (2014). https://doi.org/10.1016/j.memsci.2014.05.036
Khatuaa, C.; Chinyaa, I.; Sahab, D.; Dasa, S.; Sena, R.; Dhara, A.: Modified clad optical fibre coated with PVA/TiO2 nano composite for humidity sensing application. Int. J. Smart Sens. Intell. Syst. 8, 1424–1442 (2015). https://doi.org/10.21307/ijssis-2017-813
Wu, G.; Gan, S.; Cui, L.; Xu, Y.: Preparation and characterization of PES/TiO2 composite membranes. Appl. Surf. Sci. 254, 7080–7086 (2008). https://doi.org/10.1016/j.apsusc.2008.05.221
Elmarzugi, N.A.; Adali, T.; Bentaleb, A.M.; Keleb, E.I.; Mohamed, A.T.; Hamza, A.M.: Spectroscopic characterization of PEG–DNA biocomplexes by FTIR. J. Appl. Pharm. Sci. 4, 6–10 (2014). https://doi.org/10.7324/JAPS.2014.40802
Shoja, M.; Shameli, K.; Ahmad, M.B.; Zakaria, Z.: Preparation and characterization of poly (ε-caprolactone)/TiO2 micro-composites. Dig. J. Nanomater. Biostruct. 10, 471–477 (2015)
Rahimpour, A.; Hajavi, S.; Jahanshahi, M.; Javadi, A.; Peyravi, M.: Novel thin film nanocomposite membranes incorporated with functionalized TiO2 nanoparticles for organic solvent nanofiltration. Chem. Eng. J. 241, 155–166 (2013). https://doi.org/10.1016/j.cej.2013.12.024
Ríos, A.P.; Hernández-Fernández, F.J.; Tomás-Alonso, F.; Palacios, J.M.; Gómez, D.; Rubio, M.; Víllora, G.: A SEM–EDX study of highly stable supported liquid membranes based on ionic liquids. J. Membr. Sci. 300, 88–94 (2007). https://doi.org/10.1016/j.memsci.2007.05.010
Morihama, A.C.D.; Mierzwa, J.C.: Clay nanoparticles effects on performance and morphology of poly(vinylidene fluoride) membranes. Braz. J. Chem. Eng. 31, 79–93 (2014). https://doi.org/10.1590/S0104-66322014000100009
Espinoza-Gómez, H.; Flores-López, L.Z.; Lin, S.W.; Rogel-Hernández, E.; Ramos-Olmos, R.: Synthesis and characterization of asymmetric ultrafiltration membrane made with recycled polystyrene foam and different additives. J. Chil. Chem. Soc. 53, 1–4 (2009). https://doi.org/10.4067/s0717-97072008000400015
Mbareck, C.; Nguyen, Q.T.; Alaoui, O.T.; Barillier, D.: Elaboration, characterization and application of polysulfone and polyacrylic acid blends as ultrafiltration membranes for removal of some heavy metals from water. J. Hazard. Mater. 171, 93–101 (2009). https://doi.org/10.1016/j.jhazmat.2009.05.123
Alia, N.; Sofiah, H.; Asmadi, A.; Endut, A.: Preparation and characterization of a polysulfone ultrafiltration membrane for bovine serum albumin separation: effect of polymer concentration. Desalin. Water Treat. 32, 248–255 (2011). https://doi.org/10.5004/dwt.2011.2707
Al Mayyahi, A.: TiO2 polyamide thin film nanocomposite reverses osmosis membrane for water desalination. Membranes (Basel) (2018). https://doi.org/10.3390/membranes8030066
Fang, Y.; Duranceau, S.J.: Study of the effect of nanoparticles and surface morphology on reverse osmosis and nanofiltration membrane productivity. Membranes (Basel) 3, 196–225 (2013). https://doi.org/10.3390/membranes3030196
Pereira, V.R.; Isloor, A.M.; Bhat, U.K.; Ismail, A.F.: Preparation and antifouling properties of PVDF ultrafiltration membranes with polyaniline (PANI) nanofibers and hydrolysed PSMA (H-PSMA) as additives. Desalination 351, 220–227 (2014). https://doi.org/10.1016/j.desal.2014.08.002
Acknowledgement
The authors would like to acknowledge National Institute of Technology, Calicut, for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nivedita, S., Joseph, S. Optimization of Process Parameters using Response Surface Methodology for PCL based Biodegradable Composite Membrane for Water Purification. Arab J Sci Eng 45, 7347–7360 (2020). https://doi.org/10.1007/s13369-020-04530-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-04530-6