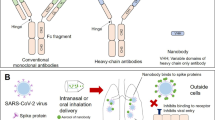
Abstract
Vaccines are the cornerstone of world health. The majority of vaccines are formulated as injectable products, facing the drawbacks of cold chain transportation, needle-stick injuries, and primary systemic immunity. Inhalable vaccines exhibited unique advantages due to their small dose, easy to use, quick effect, and simultaneous induction of mucosal and systemic responses. Facing global pandemics, especially the coronavirus disease 2019 (COVID-19), a majority of inhalable vaccines are in preclinical or clinical trials. A better understanding of advanced delivery technologies of inhalable vaccines may provide new scientific insights for developing inhalable vaccines. In this review article, detailed immune mechanisms involving mucosal, cellular, and humoral immunity were described. The preparation methods of inhalable vaccines were then introduced. Advanced nanotechnologies of inhalable vaccines containing inhalable nucleic acid vaccines, inhalable adenovirus vector vaccines, novel adjuvant–assisted inhalable vaccines, and biomaterials for inhalable vaccine delivery were emphatically discussed. Meanwhile, the latest clinical progress in inhalable vaccines for COVID-19 and tuberculosis was discussed.
Graphical Abstract






Similar content being viewed by others
Availability of data and materials
Not applicable.
References
Piot P, Larson HJ, O’Brien KL, N’kengasong J, Ng E, Sow S, et al. Immunization: vital progress, unfinished agenda. Nature. 2019;575:119–29.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15.
Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, et al. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021;384:1824–35.
Jansen EM, Frijlink HW, Hinrichs WL, Ruigrok MJ. Are inhaled mRNA vaccines safe and effective? A review of preclinical studies. Expert Opin Drug Deliv. 2022;19:1471–85.
Rockx B, Kuiken T, Herfst S, Bestebroer T, Lamers MM, Oude Munnink BB, et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020;368:1012–5.
Fraser R, Orta-Resendiz A, Mazein A, Dockrell DH. Upper respiratory tract mucosal immunity for SARS-CoV-2 vaccines. Trends Mol Med. 2023;29:255–67.
Lavelle EC, Ward RW. Mucosal vaccines - fortifying the frontiers. Nat Rev Immunol. 2022;22:236–50.
Ejemel M, Li Q, Hou S, Schiller ZA, Tree JA, Wallace A, et al. A cross-reactive human IgA monoclonal antibody blocks SARS-CoV-2 spike-ACE2 interaction. Nat Commun. 2020;11:4198.
Xu F, Wu S, Yi L, Peng S, Wang F, Si W, et al. Safety, mucosal and systemic immunopotency of an aerosolized adenovirus-vectored vaccine against SARS-CoV-2 in rhesus macaques. Emerg Microbes Infect. 2022;11:438–41.
Baker JR, Farazuddin M, Wong PT, O’Konek JJ. The unfulfilled potential of mucosal immunization. J Allergy Clin Immunol. 2022;150:1–11.
Tang J, Zeng C, Cox TM, Li C, Son YM, Cheon IS, et al. Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci Immunol. 2022;7:eadd4853.
Oh JE, Song E, Moriyama M, Wong P, Zhang S, Jiang R, et al. Intranasal priming induces local lung-resident B cell populations that secrete protective mucosal antiviral IgA. Sci Immunol. 2021;6:eabj5129.
Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101.
Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song J-Y, et al. T cell memory. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346:101–5.
Amezcua Vesely MC, Pallis P, Bielecki P, Low JS, Zhao J, Harman CCD, et al. Effector TH17 cells give rise to long-lived TRM cells that are essential for an immediate response against bacterial infection. Cell. 2019;178:1176-1188.e15.
Knight FC, Gilchuk P, Kumar A, Becker KW, Sevimli S, Jacobson ME, et al. Mucosal immunization with a pH-responsive nanoparticle vaccine induces protective CD8+ lung-resident memory T cells. ACS Nano. 2019;13:10939–60.
Rakhra K, Abraham W, Wang C, Moynihan KD, Li N, Donahue N, et al. Exploiting albumin as a mucosal vaccine chaperone for robust generation of lung-resident memory T cells. Sci Immunol. 2021;6:eabd8003.
Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–61.
Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35:161–8.
Liu G, Zhu M, Zhao X, Nie G. Nanotechnology-empowered vaccine delivery for enhancing CD8+ T cells-mediated cellular immunity. Adv Drug Deliv Rev. 2021;176:113889.
Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–3.
Laidlaw BJ, Zhang N, Marshall HD, Staron MM, Guan T, Hu Y, et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity. 2014;41:633–45.
Akkaya M, Kwak K, Pierce SK. B cell memory: building two walls of protection against pathogens. Nat Rev Immunol. 2020;20:229–38.
Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2022;40:413–42.
Lu LL, Suscovich TJ, Fortune SM, Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2018;18:46–61.
Porsch F, Mallat Z, Binder CJ. Humoral immunity in atherosclerosis and myocardial infarction: from B cells to antibodies. Cardiovasc Res. 2021;117:2544–62.
Li Y, Wang G, Li N, Wang Y, Zhu Q, Chu H, et al. Structural insights into immunoglobulin M. Science. 2020;367:1014–7.
Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claër L, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13:eabd2223.
Sudduth ER, Trautmann-Rodriguez M, Gill N, Bomb K, Fromen CA. Aerosol pulmonary immune engineering. Adv Drug Deliv Rev. 2023;199:114831.
Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67–74.
Chow MYT, Chang RYK, Chan H-K. Inhalation delivery technology for genome-editing of respiratory diseases. Adv Drug Deliv Rev. 2021;168:217–28.
Pleasants RA, Hess DR. Aerosol delivery devices for obstructive lung diseases. Respir Care. 2018;63:708–33.
Miao H, Huang K, Li Y, Li R, Zhou X, Shi J, et al. Optimization of formulation and atomization of lipid nanoparticles for the inhalation of mRNA. Int J Pharm. 2023;640:123050.
Watts AB, McConville JT, Williams RO. Current therapies and technological advances in aqueous aerosol drug delivery. Drug Dev Ind Pharm. 2008;34:913–22.
Gomez M, Vehring R. Spray drying and particle engineering in dosage form design for global vaccines. J Aerosol Med Pulm Drug Deliv. 2022;35:121–38.
Tomar J, Patil HP, Bracho G, Tonnis WF, Frijlink HW, Petrovsky N, et al. Advax augments B and T cell responses upon influenza vaccination via the respiratory tract and enables complete protection of mice against lethal influenza virus challenge. J Control Release. 2018;288:199–211.
Authelin J-R, Rodrigues MA, Tchessalov S, Singh SK, McCoy T, Wang S, et al. Freezing of biologicals revisited: scale, stability, excipients, and degradation stresses. J Pharm Sci. 2020;109:44–61.
Gomez M, McCollum J, Wang H, Ordoubadi M, Jar C, Carrigy NB, et al. Development of a formulation platform for a spray-dried, inhalable tuberculosis vaccine candidate. Int J Pharm. 2021;593:120121.
Gomez M, McCollum J, Wang H, Bachchhav S, Tetreau I, Gerhardt A. Evaluation of the stability of a spray-dried tuberculosis vaccine candidate designed for dry powder respiratory delivery. Vaccine. 2021;39:5025–36.
Saluja V, Amorij J-P, Kapteyn JC, de Boer AH, Frijlink HW, Hinrichs WLJ. A comparison between spray drying and spray freeze drying to produce an influenza subunit vaccine powder for inhalation. J Control Release. 2010;144:127–33.
Wanning S, Süverkrüp R, Lamprecht A. Pharmaceutical spray freeze drying. Int J Pharm. 2015;488:136–53.
Qin M, Du G, Sun X. Recent advances in the noninvasive delivery of mRNA. Acc Chem Res. 2021;54:4262–71.
Tang J, Cai L, Xu C, Sun S, Liu Y, Rosenecker J, et al. Nanotechnologies in delivery of DNA and mRNA vaccines to the nasal and pulmonary mucosa. Nanomaterials (Basel). 2022;12:226.
Roy CJ, Ault A, Sivasubramani SK, Gorres JP, Wei C-J, Andersen H, et al. Aerosolized adenovirus-vectored vaccine as an alternative vaccine delivery method. Respir Res. 2011;12:153.
Kachura MA, Hickle C, Kell SA, Sathe A, Calacsan C, Kiwan R, et al. A CpG-Ficoll nanoparticle adjuvant for anthrax protective antigen enhances immunogenicity and provides single-immunization protection against inhaled anthrax in monkeys. J Immunol. 2016;196:284–97.
Muralidharan P, Malapit M, Mallory E, Hayes D, Mansour HM. Inhalable nanoparticulate powders for respiratory delivery. Nanomedicine. 2015;11:1189–99.
Hald Albertsen C, Kulkarni JA, Witzigmann D, Lind M, Petersson K, Simonsen JB. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev. 2022;188:114416.
Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6:1078–94.
Yu H, Angelova A, Angelov B, Dyett B, Matthews L, Zhang Y, et al. Real-time pH-dependent self-assembly of ionisable lipids from COVID-19 vaccines and in situ nucleic acid complexation. Angew Chem Int Ed Engl. 2023;e202304977.
Angelov B, Angelova A, Filippov SK, Narayanan T, Drechsler M, Štěpánek P, et al. DNA/fusogenic lipid nanocarrier assembly: millisecond structural dynamics. J Phys Chem Lett. 2013;4:1959–64.
Qiu Y, Man RCH, Liao Q, Kung KLK, Chow MYT, Lam JKW. Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide. J Control Release. 2019;314:102–15.
Dubey A, Lobo CL, Gs R, Shetty A, Hebbar S, El-Zahaby SA. Exosomes: emerging implementation of nanotechnology for detecting and managing novel corona virus- SARS-CoV-2. Asian J Pharm Sci. 2022;17:20–34.
Li M, Qin M, Song G, Deng H, Wang D, Wang X, et al. A biomimetic antitumor nanovaccine based on biocompatible calcium pyrophosphate and tumor cell membrane antigens. Asian J Pharm Sci. 2021;16:97–109.
Kowalski PS, Rudra A, Miao L, Anderson DG. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol Ther. 2019;27:710–28.
Legere RM, Cohen ND, Poveda C, Bray JM, Barhoumi R, Szule JA, et al. Safe and effective aerosolization of in vitro transcribed mRNA to the respiratory tract epithelium of horses without a transfection agent. Sci Rep. 2021;11:371.
Patel AK, Kaczmarek JC, Bose S, Kauffman KJ, Mir F, Heartlein MW, et al. Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv Mater. 2019;31:e1805116.
Qiu Y, Chow MYT, Liang W, Chung WWY, Mak JCW, Lam JKW. From pulmonary surfactant, synthetic KL4 peptide as effective siRNA delivery vector for pulmonary delivery. Mol Pharm. 2017;14:4606–17.
Blanchard EL, Vanover D, Bawage SS, Tiwari PM, Rotolo L, Beyersdorf J, et al. Treatment of influenza and SARS-CoV-2 infections via mRNA-encoded Cas13a in rodents. Nat Biotechnol. 2021;39:717–26.
Rotolo L, Vanover D, Bruno NC, Peck HE, Zurla C, Murray J, et al. Species-agnostic polymeric formulations for inhalable messenger RNA delivery to the lung. Nat Mater. 2023;22:369–79.
Dinh P-UC, Paudel D, Brochu H, Popowski KD, Gracieux MC, Cores J, et al. Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat Commun. 2020;11:1064.
Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684–707.
Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu T, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng. 2020;4:69–83.
Popowski KD, Moatti A, Scull G, Silkstone D, Lutz H, López de Juan Abad B, et al. Inhalable dry powder mRNA vaccines based on extracellular vesicles. Matter. 2022;5:2960–74.
Zhang H, Leal J, Soto MR, Smyth HDC, Ghosh D. Aerosolizable lipid nanoparticles for pulmonary delivery of mRNA through design of experiments. Pharmaceutics. 2020;12(11):1042.
Kim J, Jozic A, Lin Y, Eygeris Y, Bloom E, Tan X, et al. Engineering lipid nanoparticles for enhanced intracellular delivery of mRNA through inhalation. ACS Nano. 2022;16:14792–806.
Lokugamage MP, Vanover D, Beyersdorf J, Hatit MZC, Rotolo L, Echeverri ES, et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat Biomed Eng. 2021;5:1059–68.
Eygeris Y, Gupta M, Kim J, Sahay G. Chemistry of lipid nanoparticles for RNA delivery. Acc Chem Res. 2022;55:2–12.
Chavda VP, Vora LK, Pandya AK, Patravale VB. Intranasal vaccines for SARS-CoV-2: from challenges to potential in COVID-19 management. Drug Discov Today. 2021;26:2619–36.
Ma D, Tian S, Qin Q, Yu Y, Jiao J, Xiong X, Guo Y, Zhang X, Ouyang X. Construction of an inhalable recombinant M2e-FP-expressing Bacillus subtilis spores-based vaccine and evaluation of its protection efficacy against influenza in a mouse model. Vaccine. 2023;41(30):4402–13.
Lee J, Arun Kumar S, Jhan YY, Bishop CJ. Engineering DNA vaccines against infectious diseases. Acta Biomater. 2018;80:31–47.
Yadav PD, Kumar S, Agarwal K, Jain M, Patil DR, Maithal K, et al. Needle-free injection system delivery of ZyCoV-D DNA vaccine demonstrated improved immunogenicity and protective efficacy in rhesus macaques against SARS-CoV-2. J Med Virol. 2023;95:e28484.
Tatlow D, Tatlow C, Tatlow S, Tatlow S. A novel concept for treatment and vaccination against Covid-19 with an inhaled chitosan-coated DNA vaccine encoding a secreted spike protein portion. Clin Exp Pharmacol Physiol. 2020;47:1874–8.
Xu Y, Yuen P-W, Lam JK-W. Intranasal DNA vaccine for protection against respiratory infectious diseases: the delivery perspectives. Pharmaceutics. 2014;6:378–415.
Kodama Y, Nakashima M, Nagahara T, Oyama N, Hashizume J, Nakagawa H, et al. Development of a DNA vaccine for melanoma metastasis by inhalation based on an analysis of transgene expression characteristics of naked pDNA and a ternary complex in mouse lung tissues. Pharmaceutics. 2020;12:E540.
Rosada RS, de la Torre LG, Frantz FG, Trombone APF, Zárate-Bladés CR, Fonseca DM, et al. Protection against tuberculosis by a single intranasal administration of DNA-hsp65 vaccine complexed with cationic liposomes. BMC Immunol. 2008;9:38.
Chen C, Han D, Cai C, Tang X. An overview of liposome lyophilization and its future potential. J Control Release. 2010;142:299–311.
Kang ML, Cho CS, Yoo HS. Application of chitosan microspheres for nasal delivery of vaccines. Biotechnol Adv. 2009;27:857–65.
Kumar US, Afjei R, Ferrara K, Massoud TF, Paulmurugan R. Gold-nanostar-chitosan-mediated delivery of SARS-CoV-2 DNA vaccine for respiratory mucosal immunization: development and proof-of-principle. ACS Nano. 2021;15:17582–601.
Schiedner G, Morral N, Parks RJ, Wu Y, Koopmans SC, Langston C, et al. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat Genet. 1998;18:180–3.
Hage E, Gerd Liebert U, Bergs S, Ganzenmueller T, Heim A. Human mastadenovirus type 70: a novel, multiple recombinant species D mastadenovirus isolated from diarrhoeal faeces of a haematopoietic stem cell transplantation recipient. J Gen Virol. 2015;96:2734–42.
Afkhami S, Yao Y, Xing Z. Methods and clinical development of adenovirus-vectored vaccines against mucosal pathogens. Mol Ther Methods Clin Dev. 2016;3:16030.
Bolinger B, Sims S, Swadling L, O’Hara G, de Lara C, Baban D, et al. Adenoviral vector vaccination induces a conserved program of CD8(+) T cell memory differentiation in mouse and man. Cell Rep. 2015;13:1578–88.
Jeyanathan M, Fritz DK, Afkhami S, Aguirre E, Howie KJ, Zganiacz A, et al. Aerosol delivery, but not intramuscular injection, of adenovirus-vectored tuberculosis vaccine induces respiratory-mucosal immunity in humans. JCI Insight. 2022;7:e155655.
Bolton DL, Song K, Tomaras GD, Rao S, Roederer M. Unique cellular and humoral immunogenicity profiles generated by aerosol, intranasal, or parenteral vaccination in rhesus macaques. Vaccine. 2017;35:639–46.
Manjaly Thomas Z-R, Satti I, Marshall JL, Harris SA, Lopez Ramon R, Hamidi A, et al. Alternate aerosol and systemic immunisation with a recombinant viral vector for tuberculosis, MVA85A: a phase I randomised controlled trial. PLoS Med. 2019;16:e1002790.
Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–201.
Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatullin AI, Shcheblyakov DV, Dzharullaeva AS, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–97.
Wu S, Huang J, Zhang Z, Wu J, Zhang J, Hu H, et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect Dis. 2021;21:1654–64.
Zhu F-C, Wurie AH, Hou L-H, Liang Q, Li Y-H, Russell JBW, et al. Safety and immunogenicity of a recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in Sierra Leone: a single-centre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2017;389:621–8.
Wu S, Zhong G, Zhang J, Shuai L, Zhang Z, Wen Z, et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat Commun. 2020;11:4081.
Pulendran B, S Arunachalam P, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov. 2021;20:454–75.
Bastola R, Noh G, Keum T, Bashyal S, Seo J-E, Choi J, et al. Vaccine adjuvants: smart components to boost the immune system. Arch Pharm Res. 2017;40:1238–48.
Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84.
Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26.
Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19:1597–608.
Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–96.
Hanna CC, Ashhurst AS, Quan D, Maxwell JWC, Britton WJ, Payne RJ. Synthetic protein conjugate vaccines provide protection against Mycobacterium tuberculosis in mice. Proc Natl Acad Sci U S A. 2021;118:e2013730118.
Reed SG, Carter D, Casper C, Duthie MS, Fox CB. Correlates of GLA family adjuvants’ activities. Semin Immunol. 2018;39:22–9.
Rossi I, Spagnoli G, Buttini F, Sonvico F, Stellari F, Cavazzini D, et al. A respirable HPV-L2 dry-powder vaccine with GLA as amphiphilic lubricant and immune-adjuvant. J Control Release. 2021;340:209–20.
Patil HP, Murugappan S, ter Veer W, Meijerhof T, de Haan A, Frijlink HW, et al. Evaluation of monophosphoryl lipid A as adjuvant for pulmonary delivered influenza vaccine. J Control Release. 2014;174:51–62.
Zhu W, Park J, Pho T, Wei L, Dong C, Kim J, et al. ISCOMs/MPLA-aadjuvanted SDAD protein nanoparticles induce improved mucosal immune responses and cross-protection in mice. Small. 2023;e2301801.
Wang Q, Bergholz JS, Ding L, Lin Z, Kabraji SK, Hughes ME, et al. STING agonism reprograms tumor-associated macrophages and overcomes resistance to PARP inhibition in BRCA1-deficient models of breast cancer. Nat Commun. 2022;13:3022.
Patil V, Hernandez-Franco JF, Yadagiri G, Bugybayeva D, Dolatyabi S, Feliciano-Ruiz N, et al. A split influenza vaccine formulated with a combination adjuvant composed of alpha-D-glucan nanoparticles and a STING agonist elicits cross-protective immunity in pigs. J Nanobiotechnology. 2022;20:477.
Feng B, Lu X, Zhang GQ, Zhao LB, Mei D. STING agonist delivery by lipid calcium phosphate nanoparticles enhances immune activation for neuroblastoma. Acta Materia Medica. 2023;2(2):216–27.
Wang J, Li P, Yu Y, Fu Y, Jiang H, Lu M, et al. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science. 2020;367:eaau0810.
Luo J, Liu X-P, Xiong F-F, Gao F-X, Yi Y-L, Zhang M, et al. Enhancing immune response and heterosubtypic protection ability of inactivated H7N9 vaccine by using STING agonist as a mucosal adjuvant. Front Immunol. 2019;10:2274.
Walvekar P, Kumar P, Choonara YE. Long-acting vaccine delivery systems. Adv Drug Deliv Rev. 2023;198:114897.
Andrianov AK, Langer R. Polyphosphazene immunoadjuvants: historical perspective and recent advances. J Control Release. 2021;329:299–315.
Jeong H, Lee C-S, Lee J, Lee J, Hwang HS, Lee M, et al. Hemagglutinin nanoparticulate vaccine with controlled photochemical immunomodulation for pathogenic influenza-specific immunity. Adv Sci (Weinh). 2021;8:e2100118.
Wang N, Wei C, Zhang Z, Liu T, Wang T. Aluminum nanoparticles acting as a pulmonary vaccine adjuvant-delivery system (VADS) able to safely elicit robust systemic and mucosal immunity. J Inorg Organomet Polym Mater. 2020;30:4203–17.
Stillman ZS, Decker GE, Dworzak MR, Bloch ED, Fromen CA. Aluminum-based metal-organic framework nanoparticles as pulmonary vaccine adjuvants. J Nanobiotechnology. 2023;21:39.
Balke I, Zeltins A. Use of plant viruses and virus-like particles for the creation of novel vaccines. Adv Drug Deliv Rev. 2019;145:119–29.
Wang C, Zheng X, Gai W, Wong G, Wang H, Jin H, et al. Novel chimeric virus-like particles vaccine displaying MERS-CoV receptor-binding domain induce specific humoral and cellular immune response in mice. Antiviral Res. 2017;140:55–61.
Wang Z, Popowski KD, Zhu D, de Juan Abad BL, Wang X, Liu M, et al. Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine. Nat Biomed Eng. 2022;6:791–805.
Zheng B, Peng W, Guo M, Huang M, Gu Y, Wang T, et al. Inhalable nanovaccine with biomimetic coronavirus structure to trigger mucosal immunity of respiratory tract against COVID-19. Chem Eng J. 2021;418:129392.
Bai X, Zhao G, Chen Q, Li Z, Gao M, Ho W, et al. Inhaled siRNA nanoparticles targeting IL11 inhibit lung fibrosis and improve pulmonary function post-bleomycin challenge. Sci Adv. 2022;8:eabn7162.
Haque S, Pouton CW, McIntosh MP, Ascher DB, Keizer DW, Whittaker MR, et al. The impact of size and charge on the pulmonary pharmacokinetics and immunological response of the lungs to PLGA nanoparticles after intratracheal administration to rats. Nanomedicine. 2020;30:102291.
Kabiri M, Sankian M, Sadri K, Tafaghodi M. Robust mucosal and systemic responses against HTLV-1 by delivery of multi-epitope vaccine in PLGA nanoparticles. Eur J Pharm Biopharm. 2018;133:321–30.
Park K, Skidmore S, Hadar J, Garner J, Park H, Otte A, et al. Injectable, long-acting PLGA formulations: analyzing PLGA and understanding microparticle formation. J Control Release. 2019;304:125–34.
Arafa MG, Girgis GNS, El-Dahan MS. Chitosan-coated PLGA nanoparticles for enhanced ocular anti-inflammatory efficacy of atorvastatin calcium. Int J Nanomedicine. 2020;15:1335–47.
Du G, Hathout RM, Nasr M, Nejadnik MR, Tu J, Koning RI, et al. Intradermal vaccination with hollow microneedles: a comparative study of various protein antigen and adjuvant encapsulated nanoparticles. J Control Release. 2017;266:109–18.
Thomas C, Rawat A, Hope-Weeks L, Ahsan F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol Pharm. 2011;8:405–15.
Li B, Siuta M, Bright V, Koktysh D, Matlock BK, Dumas ME, et al. Improved proliferation of antigen-specific cytolytic T lymphocytes using a multimodal nanovaccine. Int J Nanomedicine. 2016;11:6103–21.
Im JS, Arora P, Bricard G, Molano A, Venkataswamy MM, Baine I, et al. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity. 2009;30:888–98.
Scherließ R, Janke J. Preparation of poly-lactic-co-glycolic acid nanoparticles in a dry powder formulation for pulmonary antigen delivery. Pharmaceutics. 2021;13:1196.
Slütter B, Bal S, Keijzer C, Mallants R, Hagenaars N, Que I, et al. Nasal vaccination with N-trimethyl chitosan and PLGA based nanoparticles: nanoparticle characteristics determine quality and strength of the antibody response in mice against the encapsulated antigen. Vaccine. 2010;28:6282–91.
Gu P, Wusiman A, Wang S, Zhang Y, Liu Z, Hu Y, et al. Polyethylenimine-coated PLGA nanoparticles-encapsulated Angelica sinensis polysaccharide as an adjuvant to enhance immune responses. Carbohydr Polym. 2019;223:115128.
Du X, Tan D, Gong Y, Zhang Y, Han J, Lv W, et al. A new poly(I:C)-decorated PLGA-PEG nanoparticle promotes Mycobacterium tuberculosis fusion protein to induce comprehensive immune responses in mice intranasally. Microb Pathog. 2022;162:105335.
Elkomy MH, Khallaf RA, Mahmoud MO, Hussein RRS, El-Kalaawy AM, Abdel-Razik A-RH, et al. Intratracheally inhalable nifedipine-loaded chitosan-PLGA nanocomposites as a promising nanoplatform for lung targeting: snowballed protection via regulation of TGF-β/β-catenin pathway in bleomycin-induced pulmonary fibrosis. Pharmaceuticals (Basel). 2021;14:1225.
Boroumand H, Badie F, Mazaheri S, Seyedi ZS, Nahand JS, Nejati M, et al. Chitosan-based nanoparticles against viral infections. Front Cell Infect Microbiol. 2021;11:643953.
Sun B, Yu S, Zhao D, Guo S, Wang X, Zhao K. Polysaccharides as vaccine adjuvants. Vaccine. 2018;36(35):5226–34.
Mathews PD, Mertins O, Angelov B, Angelova A. Cubosomal lipid nanoassemblies with pH-sensitive shells created by biopolymer complexes: a synchrotron SAXS study. J Colloid Interface Sci. 2022;607:440–50.
He M, Zhong C, Hu H, Jin Y, Chen Y, Lou K, et al. Cyclodextrin/chitosan nanoparticles for oral ovalbumin delivery: preparation, characterization and intestinal mucosal immunity in mice. Asian J Pharm Sci. 2019;14:193–203.
Zhuo S-H, Wu J-J, Zhao L, Li W-H, Zhao Y-F, Li Y-M. A chitosan-mediated inhalable nanovaccine against SARS-CoV-2. Nano Res. 2022;15:4191–200.
Tabynov K, Solomadin M, Turebekov N, Babayeva M, Fomin G, Yadagiri G, et al. An intranasal vaccine comprising SARS-CoV-2 spike receptor-binding domain protein entrapped in mannose-conjugated chitosan nanoparticle provides protection in hamsters. Sci Rep. 2023;13:12115.
Ebensen T, Arntz A, Schulze K, Hanefeld A, Guzmán CA, Scherließ R. Pulmonary application of novel antigen-loaded chitosan nano-particles co-administered with the mucosal adjuvant C-Di-AMP resulted in enhanced immune stimulation and dose sparing capacity. Pharmaceutics. 2023;15:1238.
Alfagih IM, Kaneko K, Kunda NK, Alanazi F, Dennison SR, Tawfeek HM, et al. In vitro characterization of inhalable cationic hybrid nanoparticles as potential vaccine carriers. Pharmaceuticals (Basel). 2021;14:164.
Mohamed A, Pekoz AY, Ross K, Hutcheon GA, Saleem IY. Pulmonary delivery of Nanocomposite Microparticles (NCMPs) incorporating miR-146a for treatment of COPD. Int J Pharm. 2019;569:118524.
COVID-19 Vaccine Tracker. Vaccines candidates by trial phase. 2022. https://covid19.trackvaccines.org/vaccines. Accessed 22 Dec 2022.
Satti I, Meyer J, Harris SA, Manjaly Thomas Z-R, Griffiths K, Antrobus RD, et al. Safety and immunogenicity of a candidate tuberculosis vaccine MVA85A delivered by aerosol in BCG-vaccinated healthy adults: a phase 1, double-blind, randomised controlled trial. Lancet Infect Dis. 2014;14:939–46.
Tomar J, Tonnis WF, Patil HP, de Boer AH, Hagedoorn P, Vanbever R, et al. Pulmonary immunization: deposition site is of minor relevance for influenza vaccination but deep lung deposition is crucial for hepatitis B vaccination. Acta Pharm Sin B. 2019;9(6):1231–40.
Author information
Authors and Affiliations
Contributions
Li Qin: conceptualization, writing—original draft, writing—review and editing. Yanhua Sun: writing—review and editing. Nan Gao: writing—review and editing. Guixia Ling: supervision and project administration. Peng Zhang: supervision and project administration.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agreed with the content and all gave consent to submit the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, L., Sun, Y., Gao, N. et al. Nanotechnology of inhalable vaccines for enhancing mucosal immunity. Drug Deliv. and Transl. Res. 14, 597–620 (2024). https://doi.org/10.1007/s13346-023-01431-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-023-01431-7