Abstract
Triptolide (TPL) has been employed to treat hepatocellular carcinoma (HCC). However, the poor water solubility of TPL restricts its applications. Therefore, we prepared TPL-loaded cyclodextrin-based metal–organic framework (TPL@CD-MOF) to improve the solubility and bioavailability of TPL, thus enhancing the anti-tumor effect on HCC. The BET surface and the pore size of TPL@CD-MOF were 10.4 m2·g−1 and 1.1 nm, respectively. The results of XRD indicated that TPL in TPL@CD-MOF was encapsuled. TPL@CD-MOF showed a slower release than free TPL in vitro. Moreover, the CD-MOF improved the bioavailability of TPL. TPL@CD-MOF showed slightly higher, but statistically significant, anti-tumor efficacy in vitro and in vivo compared to free TPL. In addition, TPL@CD-MOF exhibited a modest improvement of the anti-tumor effects, which may be associated to the enhanced in vivo absorption. Overall, these findings suggested the potential CD-MOF as oral drug delivery carriers for anti-tumor drugs.
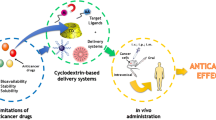
Graphical abstract
The process of TPL loading into CD-MOF and its enhanced oral bioavailability and anti-tumor activity.








Similar content being viewed by others
Availability of data and materials
All data are fully available without restriction.
References
Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–62.
Chen H, Wong CC, Liu D, Go M, Wu B, Peng S, et al. APLN promotes hepatocellular carcinoma through activating PI3K/Akt pathway and is a druggable target. Theranostics. 2019;9:5246–60.
Fares N, Peron JM. Epidemiology, natural history, and risk factors of hepatocellular carcinoma. Rev Prat. 2013;63(216–217):220–2.
Zhang X, Liu T, Li Z, Feng Y, Corpe C, Liu S, et al. Hepatomas are exquisitely sensitive to pharmacologic ascorbate (P-AscH(-)). Theranostics. 2019;9:8109–26.
Alsaied OA, Sangwan V, Banerjee S, Krosch TC, Chugh R, Saluja A, et al. Sorafenib and triptolide as combination therapy for hepatocellular carcinoma. Surgery. 2014;156:270–9.
Li X, Lu Q, Xie W, Wang Y, Wang G. Anti-tumor effects of triptolide on angiogenesis and cell apoptosis in osteosarcoma cells by inducing autophagy via repressing Wnt/beta-Catenin signaling. Biochem Biophys Res Commun. 2018;496:443–9.
Chen F, Liu Y, Wang S, Guo X, Shi P, Wang W, et al. Triptolide, a Chinese herbal extract, enhances drug sensitivity of resistant myeloid leukemia cell lines through downregulation of HIF-1alpha and Nrf2. Pharmacogenomics. 2013;14:1305–17.
Lin C, Zhang X, Chen H, Bian Z, Zhang G, Riaz MK, et al. Dual-ligand modified liposomes provide effective local targeted delivery of lung-cancer drug by antibody and tumor lineage-homing cell-penetrating peptide. Drug Deliv. 2018;25:256–66.
Liu H, Tang L, Li X, Li H. Triptolide inhibits vascular endothelial growth factor-mediated angiogenesis in human breast cancer cells. Exp Ther Med. 2018;16:830–6.
Li SG, Shi QW, Yuan LY, Qin LP, Wang Y, Miao YQ, et al. C-Myc-dependent repression of two oncogenic miRNA clusters contributes to triptolide-induced cell death in hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2018;37:51.
Ling D, Xia H, Park W, Hackett MJ, Song C, Na K, et al. pH-sensitive nanoformulated triptolide as a targeted therapeutic strategy for hepatocellular carcinoma. ACS Nano. 2014;8:8027–39.
Zhang L, Chen Y, Shi R, Rang T, Pang G, Wang B, et al. Synthesis of hollow nanocages MOF-5 as drug delivery vehicle to solve the load-bearing problem of insoluble antitumor drug oleanolic acid (OA). Inorg Chem Commun. 2018;96:20–3.
Ren Q, Li M, Deng Y, Lu A, Lu J. Triptolide delivery: Nanotechnology-based carrier systems to enhance efficacy and limit toxicity. Pharmacol Res. 2021;165:105377. https://doi.org/10.1016/j.phrs.2020.105377.
Kritskiy I, Volkova T, Surov A, Terekhova I. gamma-Cyclodextrin-metal organic frameworks as efficient microcontainers for encapsulation of leflunomide and acceleration of its transformation into teriflunomide. Carbohydr Polym. 2019;216:224–30.
Moussa Z, Hmadeh M, Abiad MG, Dib OH, Patra D. Encapsulation of curcumin in cyclodextrin-metal organic frameworks: Dissociation of loaded CD-MOFs enhances stability of curcumin. Food Chem. 2016;212:485–94.
Hartlieb KJ, Ferris DP, Holcroft JM, Kandela I, Stern CL, Nassar MS, et al. Encapsulation of Ibuprofen in CD-MOF and Related Bioavailability Studies. Mol Pharm. 2017;14:1831–9.
He Y, Hou X, Guo J, He Z, Guo T, Liu Y, et al. Activation of a gamma-cyclodextrin-based metal-organic framework using supercritical carbon dioxide for high-efficient delivery of honokiol. Carbohydr Polym. 2020;235:115935.
Chen G, Luo J, Cai M, Qin L, Wang Y, Gao L, et al. Investigation of Metal-Organic Framework-5 (MOF-5) as an Antitumor Drug Oridonin Sustained Release Carrier. Molecules. 2019; 24.
Bao Y, Yin M, Hu X, Zhuang X, Sun Y, Guo Y, et al. A safe, simple and efficient doxorubicin prodrug hybrid micelle for overcoming tumor multidrug resistance and targeting delivery. J Control Release. 2016;235:182–94.
Gu Y, Tang X, Yang M, Yang D, Liu J. Transdermal drug delivery of triptolide-loaded nanostructured lipid carriers: Preparation, pharmacokinetic, and evaluation for rheumatoid arthritis. Int J Pharm. 2019;554:235–44.
Duran-Lobato M, Martin-Banderas L, Goncalves LMD, Fernandez-Arevalo M, Almeida AJ. Comparative study of chitosan- and PEG-coated lipid and PLGA nanoparticles as oral delivery systems for cannabinoids. J Nanopart Res. 2015; 17.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 81903141, No. 81973275) and Shanghai Sailing Program (No. 20YF1424000).
Author information
Authors and Affiliations
Contributions
LZ, YG, and YY designed and participated equally all the experiments and article writing and should be considered as co-first authors. WR, WY, WJ, YM, and GC helped analyze the data and participated in some experiments.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The animal experiment was approved by the Ethics Committee of Ninth People’s Hospital, affiliated with Shanghai Jiao Tong University School of Medicine before the research (approval number SH9H-2020-A144-1).
Consent for publication
All authors have read and approved the final manuscript for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Yang, G., Wang, R. et al. γ-Cyclodextrin metal–organic framework as a carrier to deliver triptolide for the treatment of hepatocellular carcinoma. Drug Deliv. and Transl. Res. 12, 1096–1104 (2022). https://doi.org/10.1007/s13346-021-00978-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-00978-7