Abstract
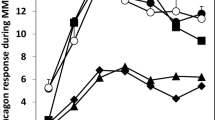
We encountered a 55-year-old Japanese man with advanced renal cell carcinoma and slowly progressive type 1 diabetes mellitus (SPT1DM), whose insulin secretory capacity was drastically reduced for a brief period after only one cycle of immune checkpoint inhibitor (ICI) treatment. The patient had been diagnosed with type 2 diabetes at the age of 53 years and was treated using oral hypoglycemic agents. However, 2 years later, he was diagnosed with SPT1DM and autoimmune thyroiditis, based on the presence of anti-glutamic acid decarboxylase antibodies (GADA) and thyroid autoantibodies, which was accompanied by advanced renal cell carcinoma. At that time, his insulin secretory capacity was preserved (CPR 2.36 ng/mL), and good glycemic control was maintained using only medical nutrition therapy (HbA1c 6.3%). He subsequently developed destructive thyroiditis approximately 2 weeks after the first cycle of ICI treatment using nivolumab (a programmed cell death-1 inhibitor) and ipilimumab (a cytotoxic T-lymphocyte-associated antigen-4 inhibitor) for advanced renal cell carcinoma. Three weeks later, his plasma glucose level markedly increased, and we detected absolute insulin deficiency and hypothyroidism. Human leukocyte antigen (HLA) analysis revealed haplotypes indicating susceptibility to type 1 diabetes mellitus (T1DM) or autoimmune thyroiditis (HLA genotype, DRB1-DQB1 *09:01–*03:03/*08:03–*06:01). He showed a good antitumor response and is currently receiving permanent insulin therapy and levothyroxine replacement with the ICI treatment. Based on this case and the available literature, patients with preexisting islet autoantibodies or SPT1DM/LADA, plus a genetic predisposition to T1DM, may have an extremely high risk of developing ICI-related T1DM for a brief period after starting ICI treatment.
Similar content being viewed by others
References
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5.
Azoury SC, Straughan DM, Shukla V. Immune checkpoint inhibitors for cancer therapy: Clinical efficacy and safety. Curr Cancer Drug Targets. 2015;15:452–62.
Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–68.
Quandt Z, Young A, Anderson M. Immune checkpoint inhibitor diabetes mellitus: a novel form of autoimmune diabetes. Clin Exp Immunol. 2020;200:131–40.
Akturk HK, Kahramangil D, Sarwal A, Hoffecker L, Murad MH, Michels AW. Immune checkpoint inhibitor-induced Type 1 diabetes: a systematic review and meta-analysis. Diabet Med. 2019;36:1075–81.
de Filette JMK, Pen JJ, Decoster L, Vissers T, Bravenboer B, Van der Auwera BJ, Gorus FK, Roep BO, Aspeslagh S, Neyns B, Velkeniers B, Kharagjitsingh AV. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur J Endocrinol. 2019;181:363–74.
Baden MY, Imagawa A, Abiru N, Awata T, Ikegami H, Uchigata Y, Oikawa Y, Osawa H, Kajio H, Kawasaki E, Kawabata Y, Kozawa J, Shimada A, Takahashi K, Tanaka S, Chujo D, Fukui T, Miura J, Yasuda K, Yasuda H, Kobayashi T, Hanafusa T. Characteristics and clinical course of type 1 diabetes mellitus related to anti-programmed cell death-1 therapy. Diabetol Int. 2019;10:58–66.
Clotman K, Janssens K, Specenier P, Weets I, De Block CEM. Programmed cell death-1 inhibitor-induced type 1 diabetes mellitus. J Clin Endocrinol Metab. 2018;103:3144–54.
Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, Gettinger S, Sznol M, Young A, Rushakoff R, Lee J, Bluestone JA, Anderson M, Herold KC. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. 2018;67:1471–80.
Tsang VHM, McGrath RT, Clifton-Bligh RJ, Scolyer RA, Jakrot V, Guminski AD, Long GV, Menzies AM. Checkpoint inhibitor-associated autoimmune diabetes is distinct from type 1 diabetes. J Clin Endocrinol Metab. 2019;104:5499–506.
Kotwal A, Haddox C, Block M, Kudva YC. Immune checkpoint inhibitors: an emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res Care. 2019;7:e000591.
Tanaka S, Ohmori M, Awata T, Shimada A, Murao S, Maruyama T, Kamoi K, Kawasaki E, Nakanishi K, Nagata M, Fujii S, Ikegami H, Imagawa A, Uchigata Y, Okubo M, Osawa H, Kajio H, Kawaguchi A, Kawabata Y, Satoh J, Shimizu I, Takahashi K, Makino H, Iwahashi H, Miura J, Yasuda K, Hanafusa T, Kobayashi T. Committee on type 1 diabetes. Diagnostic criteria for slowly progressive insulin-dependent (type1) diabetes mellitus (SPIDDM), (2012) report by the Committee on Slowly Progressive Insulin-Dependent (Type 1) Diabetes Mellitus of the Japan Diabetes Society. Diabetol Int. 2015;6:1–7.
Stenström G, Gottsäter A, Bakhtadze E, Berger B, Sundkvist G. Latent autoimmune diabetes in adults: definition, prevalence, beta-cell function, and treatment. Diabetes. 2005;54:S68–72.
Kawabata Y, Ikegami H, Kawaguchi Y, Fujisawa T, Shintani M, Ono M, Nishino M, Uchigata Y, Lee I, OgiharaT. Asian-specific HLA haplotypes reveal heterogeneity of the contribution of HLA-DR and -DQ haplotypes to susceptibility to type 1 diabetes. Diabetes. 2002; 51:545-51.
Bingley PJ, Bonifacio E, Williams AJ, Genovese S, Bottazzo GF, Gale EA. Prediction of IDDM in the general population: strategies based on combinations of autoantibody markers. Diabetes. 1997;46:1701–10.
Bingley PJ. Clinical applications of diabetes antibody testing. J Clin Endocrinol Metab. 2010;95:25–33.
Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in diabetes. Diabetes. 2005;54:S52–61.
Tanaka S, Okubo M, Nagasawa K, Takizawa S, Ichijo M, Ichijo S, Kaneshige M, Aida K, Shimura H, Mori Y, Kobayashi T. Predictive value of titer of GAD antibodies for further progression of beta cell dysfunction in slowly progressive insulin-dependent (type 1) diabetes (SPIDDM). Diabetol Int. 2016;7:42–52.
Buzzetti R, Di Pietro S, Giaccari A, Petrone A, Locatelli M, Suraci C, Capizzi M, Arpi ML, Bazzigaluppi E, Dotta F, Bosi E. Non Insulin Requiring Autoimmune Diabetes Study Group. High titer of autoantibodies to GAD identifies a specific phenotype of adult-onset autoimmune diabetes. Diabetes Care. 2007;30:932–8.
Godwin JL, Jaggi S, Sirisena I, Sharda P, Rao AD, Mehra R, Veloski C. Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer. J Immunother Cancer. 2017;5:40.
Gauci ML, Laly P, Vidal-Trecan T, Baroudjian B, Gottlieb J, Madjlessi-Ezra N, Da Meda L, Madelaine-Chambrin I, Bagot M, Basset-Seguin N, Pages C, Mourah S, Boudou P, Lebbé C, Gautier JF. Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: a case report and literature review. Cancer Immunol Immunother. 2017;66:1399–410.
Kimbara S, Fujiwara Y, Iwama S, Ohashi K, Kuchiba A, Arima H, Yamazaki N, Kitano S, Yamamoto N, Ohe Y. Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci. 2018;109:3583–90.
Ohara N, Kobayashi M, Ikeda Y, Hoshi T, Morita S, Kanefuji T, Yagi K, Suda T, Takada T, Hasegawa G, Sato Y, Hirano K, Kosugi S. Non-insulin-dependent diabetes mellitus induced by immune checkpoint inhibitor therapy in an insulinoma-associated antigen-2 autoantibody-positive patient with advanced gastric cancer. Intern Med. 2020;59:551–6.
Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS. Prediction of type 1 diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996;45:926–33.
Borg H, Gottsäter A, Fernlund P, Sundkvist G. A 12-year prospective study of the relationship between islet antibodies and beta-cell function at and after the diagnosis in patients with adult-onset diabetes. Diabetes. 2002;51:1754–62.
Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, Mychaleckyj JC, Todd JA, Bonella P, Fear AL, Lavant E, Louey A, Moonsamy P. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes. 2008;57:1084–92.
Moriguchi M, Noso S, Kawabata Y, Yamauchi T, Harada T, Komaki K, Babaya N, Hiromine Y, Ito H, Yamagata S, Murata K, Higashimoto T, Park C, Yamamoto A, Ohno Y, Ikegami H. Clinical and genetic characteristics of patients with autoimmune thyroid disease with anti-islet autoimmunity. Metabolism. 2011;60:761–6.
Okada N, Iwama S, Okuji T, Kobayashi T, Yasuda Y, Wada E, Onoue T, Goto M, Sugiyama M, Tsunekawa T, Takagi H, Hagiwara D, Ito Y, Suga H, Banno R, Hase T, Morise M, Kanda M, Yokota K, Hashimoto N, Ando M, Fujimoto Y, Nagino M, Kodera Y, Fujishiro M, Hibi H, Sone M, Kiyoi H, Gotoh M, Ando Y, Akiyama M, Hasegawa Y, Arima H. Anti-thyroid antibodies and thyroid echo pattern at baseline as risk factors for thyroid dysfunction induced by anti-programmed cell death-1 antibodies: a prospective study. Br J Cancer. 2020;122:771–7.
Danlos FX, Voisin AL, Dyevre V, Michot JM, Routier E, Taillade L, Champiat S, Aspeslagh S, Haroche J, Albiges L, Massard C, Girard N, Dalle S, Besse B, Laghouati S, Soria JC, Mateus C, Robert C, Lanoy E, Marabelle A, Lambotte O. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur J Cancer. 2018;91:21–9.
Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, Luiten RM. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773–81.
Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, Nakagawa K. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4:374–8.
Acknowledgments
The authors thank Yohei Yamanaka and Shingo Takada of the Department of Urology, Osaka Police Hospital, for their cooperation in writing this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Tetsuyuki Yasuda has received lecture fees from Takeda Pharmaceutical Company Limited, Novartis Pharmaceuticals Corp., and Nippon Boehringer Ingelheim Co., Ltd. The other authors declare that they have no conflicts of interest.
Human rights statement and informed consent
All procedures were approved by the appropriate institutional review board (the Ethics Committee of Osaka Police Hospital, approved April 28, 2020, approval number: 1190) and complies with the Declaration of Helsinki and its amendments. Informed consent was obtained from the patient for the publication of this case report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yamaguchi, H., Miyoshi, Y., Uehara, Y. et al. Case of slowly progressive type 1 diabetes mellitus with drastically reduced insulin secretory capacity after immune checkpoint inhibitor treatment for advanced renal cell carcinoma. Diabetol Int 12, 234–240 (2021). https://doi.org/10.1007/s13340-020-00459-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-020-00459-1