Abstract
Objective
Our aims were to compare diabetic patients’ evaluations of straight 32- and tapered 34-gauge 4-mm needles for usability and preference as well as the frequency of injection adverse events during insulin self-injection and to analyze the relationship between patients’ preferences and their background characteristics including thumb force measured by manual muscle testing.
Methods
We enrolled 60 insulin-treated patients and measured their maximum thumb force. Patients were randomized into two groups (32- and 34-gauge) with reverse order of needle use: 1 week with one type of needle and the next week with the other. The usability of and preference for the needles were measured using the visual analog scale (VAS), and the frequency of injection adverse events was evaluated.
Results
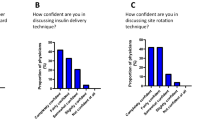
Mean maximum thumb strength was 83.5 ± 25.4 N, tended to decrease with age and was significantly lower in females than in males. The mean VAS scores regarding smooth insertion and pain during insulin delivery were significantly different, favoring the 34-gauge needle. However, the mean VAS scores regarding ease of pushing an injection button and overall preference showed no significant difference between the two needles. There was no significant difference in the frequency of injection adverse events including breaking needles.
Conclusion
Our patients had sufficient thumb force to push the injection button regardless of needle type. Although significant differences regarding smooth insertion or pain during insulin delivery were perceived, there was no difference in overall preference between the two needles, indicating the usability and safety of the two needles are not different in clinical use.


Similar content being viewed by others
References
Graff MR, McClanahan MA. Assessment by patients with diabetes mellitus of two insulin pen delivery systems versus a vial and syringe. Clin Ther. 1998;20:486–96.
Shelmet J, Schwartz S, Cappleman J, Peterson G, Skovlund S, Lytzen L, Nicklasson L, Liang J. Lyness W; InnoLet Study Group. Preference and resource utilization in elderly patients: innoLet versus vial/syringe. Diabetes Res Clin Pract. 2004;63:27–35.
Wielandt JO, Niemeyer M, Hansen MR, Bucher D, Thomsen NB. FlexTouch: a prefilled insulin pen with a novel injection mechanism with consistent high accuracy at low- (1 U), medium- (40 U), and high- (80 U) dose settings. J Diabetes Sci Technol. 2011;5:1195–9.
Schwartz S, Hassman D, Shelmet J, Sievers R, Weinstein R, Liang J, Lyness W. A multicenter, open-label, randomized, two-period crossover trial comparing glycemic control, satisfaction, and preference achieved with a 31 gauge-6 mm needle versus a 29 gauge-12.7 mm needle in obese patients with diabetes mellitus. Clin Ther. 2004;26:1663–78.
Miyakoshi M, Kamoi K, Iwanaga M, Hoshiyama A, Yamada A. Comparison of patient’s preference, pain perception, and usability between Micro Fine Plus® 31-gauge needle and microtapered NanoPass® 33-gauge needle for insulin therapy. J Diabetes Sci Technol. 2007;1:718–24.
Iwanaga M, Kamoi K. Patient perceptions of injection pain and anxiety: a comparison of NovoFine 32-gauge tip 6 mm and Micro Fine Plus 31-gauge 5 mm needles. Diabetes Technol Ther. 2009;11:81–6.
Hirsch LJ, Klaff LJ, Bailey TS, Qu S, Kassler-Taub K, Klaff LJ, Bailey TS. Comparative glycemic control, safety and patient ratings for a new 4 mm × 32G insulin pen needle in adults with diabetes. Curr Med Res Opin. 2010;26:1531–41.
Gibney MA, Arce CH, Byron KJ, Hirsch LJ. Skin and Subcutaneous adipose layer thickness in adults with diabetes at site used for insulin injection: implications for needle length recommendations. Curr Med Res Opin. 2010;26:1519–30.
Lo Presti D, Ingegnosi C, Strauss K. Skin and subcutaneous thickness at injecting sites in children with diabetes: ultrasound findings and recommendations for giving injection. Pediatr Diabetes. 2012;13:525–33.
Hirose T, Ogihara T, Tozaka S, Kanderian S, Watada H. Identification and comparison of insulin pharmacokinetics injected with a new 4-mm needle vs 6- and 8-mm needles accounting for endogenous insulin and C-peptide secretion kinetics in non-diabetic adult males. J Diabetes Invest. 2013;4:287–96.
Frid A, Hirsch LJ, Gaspar R, Hicks D, Kreugel G, Liersch J, Letondeur C, Sauvanet JP, Tubiana-Rufi N, Strauss K. New injection recommendations for patients with diabetes. Diabetes Metab. 2010;36:S3–18.
Grassi G, Scuntero P, Trepiccioni R, Marubbi F, Strauss K. Optimizing insulin injection technique and its effect on blood glucose control. J Clin Transl Endocrinol. 2014;1:145–50.
Hirsch LJ, Gibney MA, Berube J, Manocchio J. Impact of a modified needle tip geometry on penetration force as well as acceptability, preference, and perceived pain in subjects with diabetes. J Diabetes Sci Technol. 2012;6:328–35.
Aronson R, Gibney MA, Oza K, Bérubé J, Kassler-Taub K, Hirsch L. Insulin pen needles: effects of extra-thin wall needle technology on preference, confidence, and other patient ratings. Clin Ther. 2013;35:923–33.
HOGGAN scientific LLC. http://www.hogganhealth.net/microfet2.php. MicroFET2. Accessed 14 Sep 2015.
Revill SI, Robinson JO, Rosen M, Hogg MI. The reliability of a linear analogue scale for evaluating pain. Anesthesia. 1976;31:1191–8.
Mantha S, Thisted R, Foss J, Ellis JE, Roizen MF. A proposal to use confidence intervals for visual analog scale data for pain measurement to determine clinical significance. Anesth Analg. 1993;77:1041–7.
Nagai Y, Ohshige T, Arai K, Kobayashi H, Sada Y, Ohmori S, Furukawa K, Kato H, Kawata T, Ohta A, Tanaka Y. Comparison between shorter straight and thinner microtapered insulin injection needles. Diabetes Technol Ther. 2013;15:550–5.
Toraishi K, Yuizono Y, Nakamura N, Kato S, Aoki T, Ashida K, Sako Y. Force requirements and insulin delivery profiles of four injection devices. Diabetes Technol Ther. 2005;7:629–35.
Kato K. Basic study on penetration force, injection resistance and force of injection needles “BD Microfine Plus PentaPoint and Nanopass pen needle”. Jpn J Med Pharm Sci. 2013;69:115–24 (in Japanese).
Miwa T, Itoh R, Kobayashi T, Tanabe T, Shikuma J, Takahashi T, Odawara M. Comparison of the effects of a new 32-gauge × 4-mm pen needle and a 32-gauge × 6-mm pen needle on glycemic control, safety, and patient ratings in Japanese adults with diabetes. Diabetes Technol Ther. 2012;14:1084–90.
Hemmingsen H, Niemeyer M, Hansen MR, Bucher D, Thomsen NB. A prefilled insulin pen with a novel injection mechanism and a lower injection force than other prefilled insulin pens. Diabetes Technol Ther. 2011;13:1207–11.
Acknowledgments
The authors are indebted to Dr. J. Kuri, S. Tozaka and H. Tateno (Nippon Becton-Dickinson Co., Ltd.) and Dr. L. J. Hirsch (Becton-Dickinson and Co.), whose suggestions made an enormous contribution to this study. All materials for this study were provided by Nippon Becton-Dickinson Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was conducted with sponsorship of Nippon Becton-Dickinson Co., Ltd. There is no other relevant conflict of interest.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute for it was obtained from all patients for being included in the study.
About this article
Cite this article
Yamada, S., Yamada, Y., Tsukamoto, Y. et al. A comparison study of patient ratings and safety of 32- and 34-gauge insulin pen needles. Diabetol Int 7, 259–265 (2016). https://doi.org/10.1007/s13340-015-0242-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-015-0242-y