Abstract
Background and Objective
The oral first-pass metabolism is a crucial factor that plays a key role in a drug’s pharmacokinetic profile. Prediction of the oral first-pass metabolism based on chemical structural parameters can be useful in the drug-design process. Developing an orally administered drug with an acceptable pharmacokinetic profile is necessary to reduce the cost and time associated with evaluating the extent of the first-pass metabolism of a candidate compound in preclinical studies. The aim of this study is to estimate the first-pass metabolism of an orally administered drug.
Methods
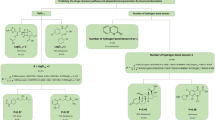
A set of compounds with reported first-pass metabolism data were collected. Moreover, human intestinal absorption percentage and oral bioavailability data were extracted from the literature to propose a classification system that split the drugs up based on their first-pass metabolism extents. Various structural parameters were calculated for each compound. The relations of the structural and physicochemical values of each compound to the class the compound belongs to were obtained using logistic regression.
Results
Initial analysis showed that compounds with logD7.4 > 1 or a rugosity factor of > 1.5 are more likely to have high first-pass metabolism. Four different models that can predict the oral first-pass metabolism with acceptable error were introduced. The overall accuracies of the models were in the range of 72% (for models with simple descriptors) to 78% (for models with complex descriptors). Although the models with simple descriptors have lower accuracies compared to complex models, they are more interpretable and easier for researchers to utilize.
Conclusion
A novel classification of drugs based on the extent of the oral first-pass metabolism was introduced, and mechanistic models were developed to assign candidate compounds to the appropriate proposed classes.




Similar content being viewed by others
References
Hurst S, Loi CM, Brodfuehrer J, El-Kattan A. Impact of physiological, physicochemical and biopharmaceutical factors in absorption and metabolism mechanisms on the drug oral bioavailability of rats and humans. Expert Opin Drug Metab Toxicol. 2007;3(4):469–89. https://doi.org/10.1517/17425255.3.4.469.
Nishimuta H, Sato K, Yabuki M, Komuro S. Prediction of the intestinal first-pass metabolism of CYP3A and UGT substrates in humans from in vitro data. Drug Metab Pharmacokinet. 2011;26(6):592–601. https://doi.org/10.2133/dmpk.DMPK-11-RG-034.
Yang J, Jamei M, Yeo KR, Tucker GT, Rostami-Hodjegan A. Prediction of intestinal first-pass drug metabolism. Curr Drug Metab. 2007;8(7):676–84. https://doi.org/10.2174/138920007782109733.
Kim MT, Sedykh A, Chakravarti SK, Saiakhov RD, Zhu H. Critical evaluation of human oral bioavailability for pharmaceutical drugs by using various cheminformatics approaches. Pharm Res. 2014;31(4):1002–14. https://doi.org/10.1007/s11095-013-1222-1.
Thörn HA. First-pass intestinal metabolism of drugs: experiences from in vitro, in vivo and simulation studies. Uppsala: Acta Universitatis Upsaliensis; 2012.
Klassen CD. Casarett & Doull’s toxicology: the basic science of poisons. 9th ed. New York: McGraw Hill; 2019. p. 209.
Pond SM, Tozer TN. First-pass elimination basic concepts and clinical consequences. Clin Pharmacokinet. 1984;9(1):1–25. https://doi.org/10.2165/00003088-198409010-00001.
Gertz M, Harrison A, Houston JB, Galetin A. Prediction of human intestinal first-pass metabolism of 25 CYP3A substrates from in vitro clearance and permeability data. Drug Metab Dispos. 2010;38(7):1147–58. https://doi.org/10.1124/dmd.110.032649.
Zgair A, Dawood Y, Ibrahem SM, Back HM, Kagan L, Gershkovich P, Lee JB. Predicting intestinal and hepatic first-pass metabolism of orally administered testosterone undecanoate. Appl Sci. 2020;10(20):1–11. https://doi.org/10.3390/app10207283.
Kaboudi N, Alizadeh AA, Shayanfar A. In silico models to predict tubular secretion or reabsorption clearance pathway using physicochemical properties and structural characteristics. Xenobiotica. 2022;52(4):346–52. https://doi.org/10.1080/00498254.2022.2076632.
KnowledgeDose. Drugs undergoing extensive first-pass metabolism. https://www.knowledgedose.com/drugs-undergoing-extensive-first-pass-metabolism/. Accessed Nov 2023.
Anzenbacher P, Zanger UM, editors. Metabolism of drugs and other xenobiotics. Hoboken: Wiley-VCH; 2012.
Lemke TL, Williams DA, Roche VF, Zito SW. Foye's principles of medicinal chemistry. 7th ed. Philadelphia: Lippincott Williams and Wilkins; 2012.
Wagner JG. Modeling first-pass metabolism. In: Pecile A, Rescigno A, editors. Mathematical and statistical approach to metabolism and distribution of chemicals and drugs. New York: Springer; 1988. pp. 129-49.
Niwa T. Using general regression and probabilistic neural networks to predict human intestinal absorption with topological descriptors derived from two-dimensional chemical structures. J Chem Inf Comput Sci. 2003;43(1):113–9. https://doi.org/10.1021/ci020013r.
OpenMed. Drugs with high first-pass metabolism. https://www.openmed.co.in/2022/02/drugs-with-high-first-pass-metabolism.html?m=1. Accessed Nov 2023.
Cyriac JM, James E. Switch over from intravenous to oral therapy: a concise overview. J Pharmacol Pharmather. 2014;5(2):83–7. https://doi.org/10.4103/0976-500X.130042.
Katzung BG. Basic and clinical pharmacology. 14th ed. New York: McGraw Hill; 2018. pp. 43–4.
Moda TL, Montanari CA, Andricopulo AD. Hologram QSAR model for the prediction of human oral bioavailability. Bioorg Med Chem. 2007;15(24):7738–45. https://doi.org/10.1016/j.bmc.2007.08.060.
Musther H, Olivares-Morales A, Hatley OJD, Liu B, Rostami HA. Animal versus human oral drug bioavailability: Do they correlate? Eur J Pharm Sci. 2014;57(1):280–91. https://doi.org/10.1016/j.ejps.2013.08.018.
Turner JV, Glass BD, Agatonovic-Kustrin S. Prediction of drug bioavailability based on molecular structure. Anal Chim Acta. 2003;485(1):89–102. https://doi.org/10.1016/S0003-2670(03)00406-9.
Klopman G, Stefan LR, Saiakhov RD. ADME evaluation: 2. A computer model for the prediction of intestinal absorption in humans. Eur J Pharm Sci. 2002;17(4-5):253–63. doi: https://doi.org/10.1016/S0928-0987(02)00219-1.
Pérez MAC, Sanz MB, Torres LR, Évalos RG, González MP, Díaz HG. A topological sub-structural approach for predicting human intestinal absorption of drugs. Eur J Med Chem. 2004;39(11):905–16. https://doi.org/10.1016/j.ejmech.2004.06.012.
Van de Waterbeemd H, Testa B, editors. Drug bioavailability: estimation of solubility, permeability, absorption and bioavailability. Weinheim: Wiley–VCH; 2009. pp. 459–71.
Cruciani G, Crivori P, Carrupt P-A, Testa B. Molecular fields in quantitative structure–permeation relationships: the VolSurf approach. J Mol Struct (Thoechem). 2000;503(1–2):17–30.
NIH. PubChem. https://pubchem.ncbi.nlm.nih.gov/. Accessed Nov 2023.
Cherkasov A, Muratov EN, Fourches D, Varnek A, Baskin II, Cronin M, et al. QSAR modeling: Where have you been? Where are you going to? J Med Chem. 2014;57(12):4977–5010. https://doi.org/10.1021/jm4004285.
Dearden JC, Cronin MTD, Kaiser KLE. How not to develop a quantitative structure-activity or structure-property relationship (QSAR/QSPR). SAR QSAR Environ Res. 2009;20(3–4):241–66. https://doi.org/10.1080/10629360902949567.
Berrar D. Cross-validation. In: Ranganathan S, Gribskov M, Nakai K, Schönbach C, editors. Encyclopedia of bioinformatics and computational biology: ABC of bioinformatics. Amsterdam: Elsevier; 2018. pp. 542–5.
Kiani YS, Jabeen I. Lipophilic metabolic efficiency (LipMetE) and drug efficiency indices to explore the metabolic properties of the substrates of selected cytochrome P450 isoforms. ACS Omega. 2020;5(1):179–88. https://doi.org/10.1021/acsomega.9b02344.
Jouyban A, Acree WE Jr. Michael H. Abraham and his developed parameters: various applications in medicine, chemistry and biology. Pharm Sci. 2022;28(2):170–3. https://doi.org/10.34172/ps.2022.1.
Azman M, Sabri AH, Anjani QK, Mustaffa MF, Hamid KA. Intestinal absorption study: challenges and absorption enhancement strategies in improving oral drug delivery. Pharmaceuticals. 2022;15(8):975. https://doi.org/10.3390/ph15080975.
Lawther BK, Kumar S, Krovvidi H. Blood–brain barrier. Continuing Education in Anaesthesia Critical Care & Pain. 2011;11(4):128–32. https://doi.org/10.1093/bjaceaccp/mkr018.
Waterhouse RN. Determination of lipophilicity and its use as a predictor of blood-brain barrier penetration of molecular imaging agents. Mol Imaging Biol. 2003;5(6):376–89. https://doi.org/10.1016/j.mibio.2003.09.014.
Siramshetty V, Williams J, Nguyễn ÐT, Neyra J, Southall N, Mathé E, et al. Validating ADME QSAR models using marketed drugs. SLAS Discov. 2021;26(10):1326–36. https://doi.org/10.1177/24725552211017520.
Hosey CM, Chan R, Benet LZ. BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs. AAPS J. 2016;18(1):251–60. https://doi.org/10.1208/s12248-015-9845-2.
Mohammadi SM, Shayanfar A, Emami S, Jouyban A. Effects of amount of excess solid, the type of stirring and sedimentation time on solubility of sodium phenytoin and lamotrigine. ADMET DMPK. 2018;6(4):269–78.
Bocci G, Oprea TI, Benet LZ. State of the art and uses for the Biopharmaceutics Drug Disposition Classification System (BDDCS): new additions, revisions, and citation references. AAPS J. 2022;24(2):37.
Golfar Y, Shayanfar A. Prediction of Biopharmaceutical Drug Disposition Classification System (BDDCS) by structural parameters. J Pharm Pharm Sci. 2019;22(1):247–69. https://doi.org/10.18433/jpps30271.
Palmer DS, Mitchell JBO. Is experimental data quality the limiting factor in predicting the aqueous solubility of druglike molecules? Mol Pharm. 2014;11(8):2962–72. https://doi.org/10.1021/mp500103r.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author Contributions
MAHH: data analysis and interpretation, drafting the article; AAA: data analysis; AS: design of the work, supervision of the project, critical revision of the article. All authors read and approved the final manuscript.
Funding
The research reported in this publication was supported by the Student Research Committee, Tabriz University of Medical Sciences (grant no: 73084).
Conflict of interest
Mir Amir Hossein Hosseini, Ali Akbar Alizadeh, and Ali Shayanfar have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Data Availability
All of the data associated with analyzing the descriptors, developing the equations and modeling, and the validation of the models are available as supplementary material on the journal’s website, along with the published article.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hosseini, M.A.H., Alizadeh, A.A. & Shayanfar, A. Prediction of the First-Pass Metabolism of a Drug After Oral Intake Based on Structural Parameters and Physicochemical Properties. Eur J Drug Metab Pharmacokinet (2024). https://doi.org/10.1007/s13318-024-00892-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s13318-024-00892-6