Abstract
Background and Objective
The tacrolimus concentration within peripheral blood mononuclear cells may correlate better with clinical outcomes after transplantation compared to concentrations measured in whole blood. However, intracellular tacrolimus measurements are not easily implemented in clinical practice. The prediction of intracellular concentrations based on whole-blood concentrations would be a solution for this. Therefore, the aim of this study was to describe the relationship between intracellular and whole-blood tacrolimus concentrations in a population pharmacokinetic (popPK) model.
Methods
Pharmacokinetic analysis was performed using non-linear mixed effects modelling software (NONMEM). The final model was evaluated using goodness-of-fit plots, visual predictive checks, and a bootstrap analysis.
Results
A total of 590 tacrolimus concentrations from 184 kidney transplant recipients were included in the study. All tacrolimus concentrations were measured in the first three months after transplantation. The intracellular tacrolimus concentrations (n = 184) were best described with an effect compartment. The distribution into the effect compartment was described by the steady-state whole-blood to intracellular ratio (RWB:IC) and the intracellular distribution rate constant between the whole-blood and intracellular compartments. Lean body weight was negatively correlated [delta objective function value (ΔOFV) −8.395] and haematocrit was positively correlated (ΔOFV = − 6.752) with RWB:IC, and both lean body weight and haematocrit were included in the final model.
Conclusion
We were able to accurately describe intracellular tacrolimus concentrations using whole-blood concentrations, lean body weight, and haematocrit values in a popPK model. This model may be used in the future to more accurately predict clinical outcomes after transplantation and to identify patients at risk for under- and overexposure.
Dutch National Trial Registry number NTR2226
Similar content being viewed by others
A population pharmacokinetic model was developed to describe the tacrolimus concentrations in peripheral blood mononuclear cells (PBMCs) using whole-blood concentrations. |
Lean body weight and haematocrit values influenced the PBMC:whole blood ratio. |
If more information is known about target levels for PBMC tacrolimus, this model can be used to individualize tacrolimus dosing based on predicted intracellular concentrations. |
1 Introduction
In most transplant centres around the world, tacrolimus is prescribed as part of the immunosuppressive therapy after kidney transplantation to prevent rejection. Although the introduction of tacrolimus has improved both graft and patient survival, the drug has some limitations [2]. As tacrolimus is known to have a narrow therapeutic range and a high variability between patients in its pharmacokinetics, doses are adjusted based on whole-blood pre-dose concentrations to ensure adequate exposure to the drug (i.e. therapeutic drug monitoring; TDM) [2]. However, in clinical practice, rejection and drug-related toxicity still occur when tacrolimus whole-blood concentrations are within the therapeutic range [3,4,5]. Theoretically, tacrolimus concentrations at the target site might better reflect tacrolimus’ immunosuppressive effect than tacrolimus concentrations in whole blood. As tacrolimus exerts its effect via the inhibition of calcineurin within lymphocytes, peripheral blood mononuclear cells (PBMCs) have been suggested to be a better matrix to monitor tacrolimus exposure [6, 7].
In recent years, several research groups have investigated intracellular tacrolimus measurement after transplantation. Their studies have revealed poor to moderate correlations between PBMC and whole-blood tacrolimus concentrations [8,9,10,11,12,13,14], which may be explained by a number of factors that affect the correlation between tacrolimus in whole blood and PBMCs, such as ABCB1 genotype, age, haematocrit, plasma protein concentrations, sex, and time after transplantation [13, 15, 16]. This poor correlation indicates that whole-blood tacrolimus exposure can differ from tacrolimus exposure at the target site. Since intracellular tacrolimus concentrations have been associated with both the occurrence and the grade of rejection in liver transplant recipients [14], tacrolimus measurement at the target site and adjusting the tacrolimus dose based on the intracellular concentration might improve tacrolimus treatment.
Although intracellular tacrolimus monitoring potentially can identify patients at risk for rejection, intracellular tacrolimus measurement is not easily implemented in clinical practice. The method is time consuming, and the pre-analytical work-up is complex. The development of a population pharmacokinetic model (popPK) can overcome some of these practical issues. A popPK model incorporating variables affecting the tacrolimus distribution makes it possible to predict a patient’s intracellular tacrolimus concentration based on the patient’s whole-blood concentration. This can reduce the number of intracellular tacrolimus concentrations that have to be measured. Moreover, a popPK model might enable the identification of patients with high or low intracellular to whole-blood tacrolimus concentration ratios, and thus a deviant tacrolimus distribution. This would make it possible to adjust the whole-blood target concentration based on the patient’s characteristics and simulated intracellular tacrolimus concentrations. This approach may eventually further personalize tacrolimus treatment after transplantation. The aim of this study was to describe the relationship between intracellular and whole-blood tacrolimus concentrations in renal transplant recipients.
2 Materials and Methods
2.1 Study Design and Data Collection
All patients included in this study received a kidney transplantation in the Erasmus MC and participated in a randomized controlled clinical trial (Dutch National Trial Registry number NTR2226) [1]. Only patients with available whole-blood and intracellular tacrolimus concentrations measured at three months post-transplantation were included in this analysis. Patients with an intracellular sample with a red level >2 were excluded from the analysis (more information is provided in the section on intracellular tacrolimus measurement below). Patients were treated with oral twice-daily tacrolimus (Prograft, Astellas Pharma, Leiden, the Netherlands) in combination with mycophenolic acid, prednisolone and basiliximab as induction therapy. For tacrolimus, TDM was applied by trained transplant physicians to achieve concentrations of 10.0–15.0 ng/ml in weeks 1–2, 8.0–12.0 ng/ml in weeks 3–4, and 5.0–10.0 ng/ml after week 4 post transplantation. Dose administrations, patient characteristics and clinical chemistry results were retrieved from the study database. As described previously [1, 16], biopsy-proven acute rejection (BPAR) was registered up until the third post-operative month. For cause, kidney transplant biopsies were reviewed in a blinded fashion by two independent pathologists and were graded according to the 2013 Banff classification of renal allograft rejection [17].
Genotyping of the ABCB1 1199G>A (rs2229109), ABCB1 3435C>T (rs1045642), CYP3A4*22 15389C>T (rs35599367), and CYP3A5*3 6986G>A (rs776746) single-nucleotide polymorphisms (SNPs) was performed according to standard laboratory procedures in an ISO15189-certified laboratory as described earlier [16]. DNA was extracted from peripheral-blood leucocytes using a Blood DNA kit (Qiagen, Courtaboeuf, France), after which genotyping was performed using TaqMan Assay reagents for allelic discrimination (Applied Biosystems, Courtaboeuf, France) with an Applied Biosystems 7900 thermal cycler.
Body composition parameters were calculated according to the following formulas:
-
Body mass index (BMI)
-
BMI = (weight in kg)/(height in m)2.
-
-
Ideal body weight (IBW) [18]
-
Female: IBW = 49 + [(length in cm − 152) × 0.39] × 1.7
-
Male: IBW = 52 + [(length in cm − 152) × 0.39] × 1.9.
-
-
Lean body weight (LBW) [19]
-
Female: LBW = 1.07 × weight in kg − 148 × (weight in kg/height in cm)2
-
Male: LBW = 1.1 × weight in kg − 128 × (weight in kg/height in cm)2.
-
-
Body surface area (BSA) [20]
-
BSA = √(height in cm × weight in kg/3600).
-
The Ethics Review Board of the Erasmus MC provided a waiver for the Medical Research Involving Human Subjects Act for this study (Medical Ethical Review Board number 2017–1029).
2.2 Tacrolimus Concentration Measurement
Tacrolimus concentrations in whole blood were determined by two immunoassays: the antibody-conjugated magnetic immunoassay (ACMIA) on a Dimension platform (Siemens Healthcare, NV, The Hague, the Netherlands) and the enzyme-multiplied immunoassay technique (EMIT; Siemens Healthcare NV), as described previously [1]. ACMIA and EMIT immunoassays demonstrated a high correlation (r = 0.97) [1].
For the intracellular concentrations, peripheral blood mononuclear cells (PBMCs) were isolated using a Ficoll separation technique, as previously described [16, 21]. To determine the presence of erythrocytes, which could affect the measured tacrolimus concentration, the redness of the sample was rated on a scale from 0 to 8 by visual inspection (Supplementary Fig. S1). Samples with a red level above 2 were excluded from this study to avoid an effect of the presence of erythrocytes. Tacrolimus concentrations in PBMCs were analysed using a validated liquid chromatography–tandem mass spectrometry (LC–MS/MS) method in an ISO15189-certified laboratory. Intracellular PBMC concentrations were calculated using the PBMC cell count and average cell volume [22].
2.3 Pharmacokinetic Modelling
Pharmacokinetic analysis was conducted by non-linear mixed effects modelling using NONMEM® version 7.4.4 (ICON Development Solutions, Ellicott City, MD, USA). Pirana (version 2.9.9), PsN® (version 4.9.0) and R (version 4.0.1) were used for data management and graphical diagnostics.
2.3.1 Structural Model
A population model describing the pharmacokinetics of tacrolimus in whole blood was first developed [23]. This previously published two-compartment model with first-order absorption and linear elimination from the central compartment was used as a reference model from which further structural models were developed [23]. Subsequently, intracellular concentrations were added to the dataset and linked to the structural model. As only one pre-dose concentration was available per patient, no information about the changes in intracellular concentration over time were available. We assumed a fast equilibrium between whole-blood and intracellular concentrations without saturation at higher concentrations, based on previous observations [21]. Structural models with both fixed and estimated parameters and models with and without mass transfer were tested to describe the intracellular compartment. As the intracellular data were obtained from the same patients and samples as the whole-blood model, initially the individual parameters for the whole-blood structural model were used. An additional error for the intracellular concentrations was added because of the different sample preparation.
2.3.2 Covariate Model
Covariates were selected based on their known or theoretical relationships with tacrolimus pharmacokinetics and theoretical plausibility. The following demographic, disease, and genetic characteristics were evaluated as potential model covariates: weight [total body weight (TBW), lean body weight (LBW), ideal body weight (IBW), body mass index (BMI), and body surface area (BSA)], sex, age, haematocrit, albumin, and ABCB1 genotype polymorphisms (3435C>T and 1199G>A). The relationship between covariates and inter-individual variability (IIV) was first investigated graphically and then further tested in a univariate analysis. Covariates that significantly improved the model (p ≤ 0.05) were added to the full model. A backward elimination process was then performed, with statistical significance indicated by p ≤ 0.001.
Continuous covariates were normalized to the population median values and incorporated as power model functions; see Eq. 1 below. Categorical covariates were transformed to binary covariates and incorporated as shown in Eq. 2 below.
Here, θi is the individual-model-predicted pharmacokinetic parameter (i.e. the WB:IC ratio) for an individual with covariate value covi, θpop is the population estimate for that parameter, covm represents the median covariate value, and θcov is the covariate effect. In the equation for categorical covariates, covi is either 1 or 0.
2.3.3 Model Selection and Evaluation
Model selection was based on minimum objective function value (OFV) parameter precision, error estimates, eta shrinkage values, and visual inspection of the goodness-of-fit plots. The validity of the final model was evaluated using bootstrap (by simulating 200 datasets) and visual predictive checks (VPCs, by simulating 1000 datasets) and then generating the 95% prediction intervals from these simulations.
2.3.4 Simulations
To give an illustration of the effect of the significant covariates found in the covariate analysis, deterministic simulations were performed. Whole-blood and intracellular tacrolimus pre-dose concentrations at steady state were simulated for the values of the 10th, 50th, and 90th percentiles of a specific covariate while keeping the other covariates similar to the population median.
2.4 Statistical Analysis
All analyses were performed with R (version 3.5.3). Each categorical variable is described as the number of cases with the proportion. Each continuous variable with a non-parametric distribution is described as the median with the interquartile range (IQR). The Mann–Whitney U test was used to compare non-parametric continuous variables.
3 Results
3.1 Patient Characteristics
Out of the 237 patients included in the original trial by Shuker et al. [1], 184 kidney transplant recipients were included in the present study. A total of 53 patients were excluded, because no intracellular tacrolimus concentration (n = 36) was available at month 3 post-transplantation, there were missing data (n = 8), or the red level of the intracellular samples was above 2 (n = 9; Supplementary Fig. S2). A total of 590 tacrolimus concentrations (intracellular concentrations, n = 184, whole-blood concentrations, n = 406) from 184 kidney transplant recipients were included in the study. For a complete overview of the patient and clinical characteristics, see Table 1. Their median age at time of transplantation was 57 years (IQR 46–64) and the median weight was 80.5 kg (IQR 68.8–92.1). Over a three-month period, the median serum creatinine concentration was 137 µmol/L (IQR 110–183) and haematocrit was 0.34 (0.31–0.37).
Out of the 184 patients, 10.9% were carriers of the CYP3A4*22 allele and 22.3% were carriers of the CYP3A5*1 allele. Moreover, 47.8% of the patients were heterozygous for the ABCB1 3435C>T SNP, 22.8% were homozygous for 3435CC, and 23.9% were homozygous for the 3435T allele. For 5.4%, the genotype was unknown. In the case of the ABCB1 1199G>A SNP, 6.5% were heterozygous for 1199GA, 84.8% were homozygous for GG, 1.1% were homozygous for AA, and the genotype was unknown for 7.6% of the patients. The ABCB1 3435C>T genotype was in Hardy–Weinberg equilibrium (p > 0.05), but ABCB1 1199G>A genotype was not (p = 0.04956). However, only two patients were homozygous for the variant allele (1199AA), and with expected counts below five, the chi-square approximation may be incorrect.
3.2 Structural Model
The model previously developed by Andrews et al. [23] was extended with an intracellular compartment. The intracellular concentrations of tacrolimus were best described by a pharmacokinetic model with an effect compartment without mass transfer from the central compartment (Fig. 1). The distribution into the effect compartment was described by the steady-state whole-blood to intracellular ratio RWB:IC and the intracellular distribution rate constant between the whole-blood and intracellular compartments (KWB-IC) (Fig. 1 and Eq. 3). Final parameter estimates are presented in Table 2. As only pre-dose intracellular concentrations were available, KWB-IC could not be estimated accurately. Therefore, KWB-IC was fixed at 0.9, indicating a fast equilibrium between whole-blood and PBMC concentrations. RWB:IC was 14100, indicating that, on average, there was a 14-fold higher concentration in the PBMCs compared to that in whole blood, with a large IIV of 38.9%.
Population pharmacokinetic model with intracellular compartment. CL clearance, F bioavailability of oral tacrolimus, Ka absorption rate constant, KWB-IC distribution rate constant, Q inter-compartmental clearance, RWB-IC ratio between whole-blood and intracellular tacrolimus, Tlag lag time, V1 central compartment, V2 peripheral compartment
where Aic is the amount in the intracellular PBMC compartment, Ac is the amount in the central whole-blood compartment, and Vc is the volume of distribution of the central compartment.
3.3 Covariate Model
The univariate analysis with a p threshold of 0.05 resulted in five significant covariates (TBW ΔOFV = − 6.362; LBW ΔOFV = − 8.395; IBW ΔOFV = − 8.405; BSA ΔOFV = − 7.998; haematocrit ΔOFV = − 6.752; Table 3). After backward elimination with a lower threshold of 0.001, only LBW and haematocrit remained in the final model (for a full list of the covariates tested, see Table 3). LBW was positively correlated and haematocrit negatively correlated with the WB:IC ratio (Eq. 4). The addition of LBW and haematocrit to the model decreased the IIV from 41.8 to 38.9%.
3.4 Model Evaluation
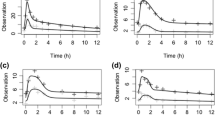
Standard goodness-of-fit plots (Fig. 2) showed no major deviations except for a small underprediction at higher concentrations. The VPC of the final model showed good predictive performance over the whole range of each covariate and over the entire range of whole-blood concentrations (Fig. 3). In the bootstrap results (Table 2), the medians of the estimated parameters were within the 95th percentile range.
Goodness-of-fit plots of the final model. A Observed intracellular tacrolimus concentrations versus predicted intracellular tacrolimus concentrations. B Observed intracellular tacrolimus concentrations versus the individual predicted intracellular tacrolimus concentrations. C The conditional weighted residuals versus the time after transplantation. D The conditional weighted residuals versus the predicted intracellular tacrolimus concentrations. CWRES conditional weighted residuals
Visual predictive checks showing how well the mean observed intracellular tacrolimus concentration (red line) versus the lean body weight (A) and the haematocrit concentration (B) falls within the 95% confidence interval for the predicted mean tacrolimus concentration (red area) and how well the variability of the observed intracellular tacrolimus concentration (red dotted line) falls within the 95% confidence interval for the predicted variability of the intracellular tacrolimus concentration (blue area)
3.5 Simulations
The whole-blood and intracellular tacrolimus concentrations were simulated for different values of the covariates that were included in the final model (Fig. 4; Supplementary Table S1). The simulations with different values of haematocrit (10th percentile: 0.28 L/L; 50th percentile: 0.34 L/L; 90th percentile: 0.43 L/L) showed a higher WB:IC ratio for higher haematocrit values. Since LBW was calculated based on the patient’s body weight, and the patient’s body weight also determines the patient’s BSA, which was included in the model of the whole-blood tacrolimus concentration, we used different values of body weight to simulate the LBW effect. With higher body weight (10th percentile: 58.5 kg; 50th percentile: 78.9 kg; 90th percentile: 102 kg), the WB:IC ratio decreased.
Simulations (n = 1000) of the tacrolimus WB:IC ratio for different haematocrit values (A) and body composition values (B). The box represents the 25th percentile, the median (middle line), and the 75th percentile. The upper whisker reaches to the highest value up to 1.5 times the interquartile range (IQR). The lower whisker reaches to the lowest value up to 1.5 times the IQR. The dots represent the concentrations that lie further away than 1.5 times the IQR. BSA body surface area, LBW lean body weight, IC intracellular, WB whole blood
3.6 Rejection
Out of the 184 patients, 16 (8.7%) developed BPAR within the first three months after kidney transplantation. Borderline rejection was diagnosed in five patients (2.7%), and rejection was presumed in four patients (2.1%) (these patients were treated, but rejection was not histologically confirmed). In one patient, the rejection status was unknown, and the other 157 patients remained rejection free. No statistically significant differences in the RWB:IC were observed between patients with (n = 16) and without (n = 158) BPAR [median 14371 (IQR 13060–17937) versus 12274 (IQR 10708–15159); p = 0.086; Fig. 5].
Boxplot of the whole-blood to intracellular tacrolimus concentration ratio for patients with and without biopsy-proven acute rejection (BPAR). The box represents the 25th percentile, the median (middle line), and the 75th percentile. The upper whisker reaches to the highest value up to 1.5 times the interquartile range (IQR). The lower whisker reaches to the lowest value up to 1.5 times the IQR. The dots represent the concentrations that lie further away than 1.5 times the IQR
4 Discussion
In the present study, a popPK model with an intracellular compartment was developed for the immunosuppressant tacrolimus in kidney transplant recipients. The model included LBW and haematocrit as covariates affecting the ratio between the whole-blood and intracellular tacrolimus concentrations.
Tacrolimus is an immunosuppressant with a narrow therapeutic range and a high inter-patient variability in its pharmacokinetics. Assuming that tacrolimus concentrations at the target site correlate better with clinical outcomes than whole-blood concentrations (which, however, remains unclear from previous studies [8, 14, 16, 24]), identifying patients with a high or low whole-blood to intracellular concentration ratio is important to avoid adverse drug-related clinical outcomes. A popPK model with an intracellular compartment might help in predicting the tacrolimus concentration at the target site and identifying patients with a particularly high or low exposure within lymphocytes, based on whole-blood tacrolimus concentrations. Moreover, popPK models can provide dosing advice for reaching a certain intracellular target exposure. Currently, no dose recommendations can be provided, as the intracellular tacrolimus target levels have not been defined. As stated in the Sect. 1, based on its mechanism of action, a correlation between intracellular tacrolimus concentrations and the efficacy of tacrolimus can be expected. However, so far, only one study has demonstrated a correlation between intracellular tacrolimus concentrations and rejection after liver transplantation [14]. In the present study, we did include an exploratory analysis of the correlation between RWB:IC and rejection, which did not show a significant correlation. However, our study was not powered to detect this. Because the lack of correlation between intracellular concentration and rejection in previous studies might be attributable to limitations of the study design and the analytical method, we believe that more research on the pharmacodynamic effect of the intracellular concentration is needed. When intracellular target concentration ranges are established, our popPK model could be used in clinical practice to predict intracellular concentrations based on whole blood measurements. This is of great clinical benefit, as measuring intracellular concentrations is time consuming and the pre-analytical work-up is complex. However, before this can be implemented in clinical practice, the results of this popPK model will need to be validated in an external cohort.
In the present analysis, LBW was one of the covariates that significantly correlated with the ratio between the tacrolimus whole-blood and intracellular concentrations. Including LBW in the model led to a lower inter-individual variability in RWB:IC, and thus it can explain some of the inter-patient variability. It also reduced the residual inter-individual variability in the intracellular tacrolimus concentrations. An explanation for the correlation between LBW and the RWB:IC might be that leucocyte differentiation, and thus the PBMC composition, correlates with the patient’s body composition parameters [25,26,27,28], since leptin has been associated with haematopoiesis and stimulates the appearance of CD34+ cells, the precursor cells for granulocytes and monocytes [26, 29]. Monocytes have a relatively large intracellular volume compared to other PBMC subsets, so the total amount of tacrolimus per individual cell may be higher in these cells compared to other PBMC subsets. As the intracellular concentration is normalized to the number of cells, an increase of monocytes might explain the decreased RWB:IC with increasing LBW. Moreover, tacrolimus is a lipophilic drug. The fat-free mass may affect the volume of distribution of tacrolimus, which may in turn affect its transfer rate into the intracellular compartment. Finally, the correlation between LBW and RWB:IC may be explained by the fact that we modelled the intracellular concentrations in an effect compartment without mass transfer. In patients with a higher LBW, this will result in an underestimation of the intracellular volume and thereby an overestimation of the intracellular concentration, causing an increase in RWB:IC.
Haematocrit was positively correlated with the WB:IC tacrolimus concentration ratio. This relationship can be explained by the high binding capacity of tacrolimus to erythrocytes [30]. As haematocrit reflects the erythrocyte concentration, a higher haematocrit might result in a higher binding capacity, which in turn decreases the shift to the intracellular compartment. This is also in line with the results of the study by Han et al. and our previous study in kidney transplant recipients [13, 16].
ABCB1 is an efflux transporter of tacrolimus that is present on the cell surfaces of lymphocytes. SNPs in the ABCB1 gene may result in altered activity of this transporter [31, 32], which may in turn affect the distribution of tacrolimus over the different compartments [33, 34]. In the present study, we found no statistically significant effect of the ABCB1 genotypes 3435C>T and 1199G>A on RWB:IC. This is in line with a previous study by Han et al. [13] in 213 kidney transplant recipients with stable renal function. In their study, gender, haematocrit, and time after transplantation significantly correlated with RWB:IC, whereas ABCB1 genotype (3435C>T, 2677G>T, or 1236C>T SNP) was not a significant predictor of RWB:IC. Moreover, in a pharmacokinetic analysis by Tron et al., ABCB1 genotype was not included as significant covariate in the popPK-PD model, although the ABCB1 2677G>T SNP and the ABCB1 haplotype (3435/1236/2677) were significantly associated with RWB:IC [12]. Also, other studies have observed a significant effect of the ABCB1 genotype on the distribution of tacrolimus. Those studies reported higher intracellular tacrolimus concentrations and intra-hepatic tacrolimus concentrations in patients with the 1199G>A, 3435C>T, 1236C>T, and 2677G>T/A polymorphisms [15, 35].
Other covariates that have been correlated with RWB:IC are gender, total plasma protein concentration, and time after transplantation [13, 15, 16]. In a previous study in our centre that included the same patient population, age, albumin, and haematocrit were significantly associated with RWB:IC at month 3 after transplantation in a multivariable regression analysis [16]. There are a few factors that can explain these slightly different findings. First, different data were used. Some patients who were excluded from the previous study could have been included in the present study, as NONMEM can take into account differences in the timing of the blood sample. Second, different methods were used to investigate the relationship between covariate factors and RWB:IC (multivariable linear regression versus non-linear mixed effects modelling). Third, other covariates were tested and found to affect the ratio.
A possible limitation of this study is that the structural model is a model without mass transfer. This was chosen since the volume of the intracellular compartment could not be estimated. This is due to the fact that the PBMCs are also part of the central compartment, and only a very small proportion of this central compartment consists of PBMCs (0.04%). This mathematical difficulty was overcome by treating the intracellular compartment as an effect compartment, for which no volume has to be estimated. This method for modelling intracellular concentrations has been described before [36]. However, by not estimating a volume for the intracellular compartment, there is no correction for the variation in the number of PBMCs in the measurement. As the intracellular concentration is described per million cells, this may also affect RWB:IC, and may in turn explain the high variability in RWB:IC. In addition, since we did not have AUCs for the intracellular tacrolimus measurements, we could not reliably estimate the distribution rate constant (KWB-IC). We knew from the literature and previous measurements in other patients that the intracellular concentrations are in fast equilibrium with the whole blood concentrations [12]. Therefore, this parameter was fixed, and simulations for pre-dose concentrations rather than for AUCs were performed. The validity of this estimate was evaluated by a sensitivity analysis, which showed no significant changes in final parameter estimates.
A limitation of the present study is that, at the time of the prospective clinical trial, tacrolimus whole-blood concentrations were measured with immunoassays rather than the more sensitive LC-MS/MS method. However, to take the variability in this method into account, an additional and proportional error for the immunoassay is included in the model for the whole-blood compartment. Second, as tacrolimus is largely bound to red blood cells, the presence of red blood cells can affect the intracellular tacrolimus concentration that is measured. However, as this effect was not known at the time of this study, no red blood cell lysing step was included in the preparation of the intracellular tacrolimus samples. To minimize the potential effect of the presence of red blood cells on the intracellular tacrolimus concentrations, samples were excluded based on the visual red level of the cell pellets. We expect that the small underestimation of individual intracellular concentrations in the high concentration range could be an effect of red blood cell contamination in these samples. The higher variability in the lower range of concentrations could be a result of imprecision of the assay in this concentration range. In future studies, it is recommended that a red blood cell washing step should be performed in PBMC isolation to purify the sample from contamination with tacrolimus bound to erythrocytes. Finally, we had only one available intracellular sample per patient. This might explain the high variability in RWB:IC. For future studies, it is recommended that multiple intracellular measurements should be included per patient.
5 Conclusion
In conclusion, a population pharmacokinetic model with an intracellular compartment was developed using data from 184 kidney transplant recipients. LBW and haematocrit significantly affected the ratio between whole-blood and intracellular tacrolimus concentrations. A dosing algorithm with an intracellular compartment could be used to estimate tacrolimus concentrations within lymphocytes based on whole-blood tacrolimus concentrations and to identify patients at risk for tacrolimus under- and overexposure at the site of action.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
The NONMEM code is included in Supplementary Data S2.
References
Shuker N, Bouamar R, van Schaik RH, Clahsen-van Groningen MC, Damman J, Baan CC, et al. A randomized controlled trial comparing the efficacy of Cyp3a5 genotype-based with body-weight-based tacrolimus dosing after living donor kidney transplantation. Am J Transplant. 2016;16(7):2085–96.
Brunet M, van Gelder T, Asberg A, Haufroid V, Hesselink DA, Langman L, et al. Therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther Drug Monit. 2019;41(3):261–307.
Bouamar R, Shuker N, Hesselink DA, Weimar W, Ekberg H, Kaplan B, et al. Tacrolimus predose concentrations do not predict the risk of acute rejection after renal transplantation: a pooled analysis from three randomized-controlled clinical trials. Am J Transplant. 2013;13(5):1253–61.
Daher Abdi Z, Prémaud A, Essig M, Alain S, Munteanu E, Garnier F, et al. Exposure to mycophenolic acid better predicts immunosuppressive efficacy than exposure to calcineurin inhibitors in renal transplant patients. Clin Pharmacol Ther. 2014;96(4):508–15.
Rodriguez-Peralvarez M, Germani G, Darius T, Lerut J, Tsochatzis E, Burroughs AK. Tacrolimus trough levels, rejection and renal impairment in liver transplantation: a systematic review and meta-analysis. Am J Transplant. 2012;12(10):2797–814.
Capron A, Haufroid V, Wallemacq P. Intra-cellular immunosuppressive drugs monitoring: a step forward towards better therapeutic efficacy after organ transplantation? Pharmacol Res. 2016;111:610–8.
van Gelder T, Klupp J, Sawamoto T, Christians U, Morris RE. ATP-binding cassette transporters and calcineurin inhibitors: potential clinical implications. Transplant Proc. 2001;33(3):2420–1.
Klaasen RA, Bergan S, Bremer S, Daleq L, Andersen AM, Midtvedt K, et al. Longitudinal study of tacrolimus in lymphocytes during the first year after kidney transplantation. Ther Drug Monit. 2018;40(5):558–66.
Pensi D, De Nicolo A, Pinon M, Calvo PL, Nonnato A, Brunati A, et al. An UPLC-MS/MS method coupled with automated on-line SPE for quantification of tacrolimus in peripheral blood mononuclear cells. J Pharm Biomed Anal. 2015;107:512–7.
Pensi D, De Nicolo A, Pinon M, Pisciotta C, Calvo PL, Nonnato A, et al. First UHPLC-MS/MS method coupled with automated online SPE for quantification both of tacrolimus and everolimus in peripheral blood mononuclear cells and its application on samples from co-treated pediatric patients. J Mass Spectrom. 2017;52(3):187–95.
Lemaitre F, Antignac M, Fernandez C. Monitoring of tacrolimus concentrations in peripheral blood mononuclear cells: application to cardiac transplant recipients. Clin Biochem. 2013;46(15):1538–41.
Tron C, Woillard JB, Houssel-Debry P, David V, Jezequel C, Rayar M, et al. Pharmacogenetic-whole blood and intracellular pharmacokinetic–pharmacodynamic (PG-PK2-PD) relationship of tacrolimus in liver transplant recipients. PLoS ONE. 2020;15(3):e0230195.
Han SS, Yang SH, Kim MC, Cho JY, Min SI, Lee JP, et al. Monitoring the intracellular tacrolimus concentration in kidney transplant recipients with stable graft function. PLoS ONE. 2016;11(4):e0153491.
Capron A, Lerut J, Latinne D, Rahier J, Haufroid V, Wallemacq P. Correlation of tacrolimus levels in peripheral blood mononuclear cells with histological staging of rejection after liver transplantation: preliminary results of a prospective study. Transpl Int. 2012;25(1):41–7.
Capron A, Mourad M, De Meyer M, De Pauw L, Eddour DC, Latinne D, et al. CYP3A5 and ABCB1 polymorphisms influence tacrolimus concentrations in peripheral blood mononuclear cells after renal transplantation. Pharmacogenomics. 2010;11(5):703–14.
Francke MI, Hesselink DA, Li Y, Koch BCP, de Wit LEA, van Schaik RHN, et al. Monitoring the tacrolimus concentration in peripheral blood mononuclear cells of kidney transplant recipients. Br J Clin Pharmacol. 2021;87(4):1918–29.
Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14(2):272–83.
Robinson JD, Lupkiewicz SM, Palenik L, Lopez LM, Ariet M. Determination of ideal body weight for drug dosage calculations. Am J Hosp Pharm. 1983;40(6):1016–9.
Waterlow JC, James WPT, editors. Research on obesity: a report of the DHSS/MRC Group, compiled by W. P. T. James for the Department of Health and Social Security and the Medical Research Council. London: HMSO; 1976.
Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098.
Bahmany S, de Wit LEA, Hesselink DA, van Gelder T, Shuker NM, Baan C, et al. Highly sensitive and rapid determination of tacrolimus in peripheral blood mononuclear cells by liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. 2019;33(1):e4416.
Simiele M, D’Avolio A, Baietto L, Siccardi M, Sciandra M, Agati S, et al. Evaluation of the mean corpuscular volume of peripheral blood mononuclear cells of HIV patients by a Coulter counter to determine intracellular drug concentrations. Antimicrob Agents Chemother. 2011;55(6):2976–8.
Andrews LM, Hesselink DA, van Schaik RHN, van Gelder T, de Fijter JW, Lloberas N, et al. A population pharmacokinetic model to predict the individual starting dose of tacrolimus in adult renal transplant recipients. Br J Clin Pharmacol. 2019;85(3):601–15.
Lemaitre F, Blanchet B, Latournerie M, Antignac M, Houssel-Debry P, Verdier MC, et al. Pharmacokinetics and pharmacodynamics of tacrolimus in liver transplant recipients: inside the white blood cells. Clin Biochem. 2015;48(6):406–11.
Purdy JC, Shatzel JJ. The hematologic consequences of obesity. Eur J Haematol. 2021;106(3):306–19.
Laharrague P, Oppert JM, Brousset P, Charlet JP, Campfield A, Fontanilles AM, et al. High concentration of leptin stimulates myeloid differentiation from human bone marrow CD34+ progenitors: potential involvement in leukocytosis of obese subjects. Int J Obes Relat Metab Disord. 2000;24(9):1212–6.
Johannsen NM, Priest EL, Dixit VD, Earnest CP, Blair SN, Church TS. Association of white blood cell subfraction concentration with fitness and fatness. Br J Sports Med. 2010;44(8):588–93.
Herishanu Y, Rogowski O, Polliack A, Marilus R. Leukocytosis in obese individuals: possible link in patients with unexplained persistent neutrophilia. Eur J Haematol. 2006;76(6):516–20.
Haas P, Straub RH, Bedoui S, Nave H. Peripheral but not central leptin treatment increases numbers of circulating NK cells, granulocytes and specific monocyte subpopulations in non-endotoxaemic lean and obese LEW-rats. Regul Pept. 2008;151(1–3):26–34.
Biagiotti S, Rossi L, Bianchi M, Giacomini E, Pierigè F, Serafini G, et al. Immunophilin-loaded erythrocytes as a new delivery strategy for immunosuppressive drugs. J Control Release. 2011;154(3):306–13.
Wang D, Johnson AD, Papp AC, Kroetz DL, Sadee W. Multidrug resistance polypeptide 1 (MDR1, ABCB1) variant 3435C>T affects mRNA stability. Pharmacogenet Genom. 2005;15(10):693–704.
Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmöller J, Johne A, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97(7):3473–8.
Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part I. Clin Pharmacokinet. 2010;49(3):141–75.
Tron C, Lemaitre F, Verstuyft C, Petitcollin A, Verdier MC, Bellissant E. Pharmacogenetics of membrane transporters of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2019;58(5):593–613.
Elens L, Capron A, Kerckhove VV, Lerut J, Mourad M, Lison D, et al. 1199G>A and 2677G>T/A polymorphisms of ABCB1 independently affect tacrolimus concentration in hepatic tissue after liver transplantation. Pharmacogenet Genom. 2007;17(10):873–83.
Kip AE, Castro MDM, Gomez MA, Cossio A, Schellens JHM, Beijnen JH, et al. Simultaneous population pharmacokinetic modelling of plasma and intracellular PBMC miltefosine concentrations in New World cutaneous leishmaniasis and exploration of exposure response relationships. J Antimicrob Chemother. 2018;73(8):2104-11.
Acknowledgements
Not applicable.
Funding
No external funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. DAH was responsible for the clinical care of the patients. RHNvS was responsible for the genotyping of the patients. LEAdW and CCB were responsible for the measurement of the whole-blood and intracellular tacrolimus concentrations. Data collection and analysis were performed by MIF, LGF, LMA, and BCMdW. The first draft of the manuscript was written by LGWF and MIF, and all authors contributed to the writing of the manuscript and commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
D.A. Hesselink has received grant support (paid to his institution) from Astellas Pharma, Chiesi Farmaceutici SpA, and Bristol Myers-Squibb, as well as lecture and consulting fees from Astellas Pharma, Chiesi Farmaceutici SpA, Novartis Pharma, and Vifor Pharma. All other authors declared no competing interests for this work.
Ethics approval
This study was a post-hoc analysis of a randomized controlled trial [1] (Dutch National Trial Registry number NTR2226). For the present study, the Ethics Review Board of the Erasmus MC provided a waiver for the Medical Research Involving Human Subjects Act for this study (Medical Ethical Review Board number 2017–1029). All study procedures were in accordance with the principles of the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all patients before inclusion in the previous trial by Shuker et al. [1].
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Franken, L.G., Francke, M.I., Andrews, L.M. et al. A Population Pharmacokinetic Model of Whole-Blood and Intracellular Tacrolimus in Kidney Transplant Recipients. Eur J Drug Metab Pharmacokinet 47, 523–535 (2022). https://doi.org/10.1007/s13318-022-00767-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-022-00767-8