Abstract
Background and Objectives
Driving under the influence of diazepam is increasing in China. The pharmacokinetics of diazepam and its metabolites, especially the glucuronide metabolites, are helpful in the identification of diazepam use by drivers. This study aimed to investigate the pharmacokinetics of diazepam and its metabolites (nordazepam, oxazepam, oxazepam glucuronide and temazepam glucuronide) in the blood of Chinese people, and to provide basic data for identifying diazepam use and estimating the time of last diazepam ingestion.
Methods
A total of 28 participants (14 men, 14 women) were recruited and each person received 5 mg diazepam orally. Whole blood was collected at 0 h (pre-dose), and 1 h, 2 h, 4 h, 8 h, 12 h, and 24 h, and at 2 days, 3 days, 6 days, 12 days, and 15 days post-dose. Analytes of interest were extracted via solid-phase extraction and analyzed by a liquid chromatography tandem mass spectrometry method operated in a positive multiple response monitoring mode. Pharmacokinetic parameters were analyzed by a pharmacokinetic software DAS according to the non-compartment model. The time of last diazepam use was estimated using the concentration ratios of diazepam to metabolites and metabolites to metabolites from controlled drug administration studies.
Results
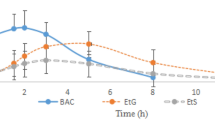
The respective time of maximum concentration, the maximum concentration and the elimination half-life of diazepam were 1.04 ± 1.00 h, 87.37 ± 31.92 ng/mL and 129.07 ± 75.00 h; of nordazepam were 133.14 ± 109.63 h, 3.80 ± 1.75 ng/mL, and 229.73 ± 236.83 h; of oxazepam were 100.29 ± 87.16 h, 1.62 ± 2.64 ng/mL, and 382.86 ± 324.58 h; of temazepam glucuronide were 44.43 ± 55.41 h, 2.08 ± 0.88 ng/mL, and 130.53 ± 72.11 h; and of oxazepam glucuronide were 66.86 ± 56.33 h, 1.10 ± 0.41 ng/mL, and 240.66 ± 170.12 h. A good correlation model was obtained from the concentration ratio of diazepam to nordazepam and the time of diazepam use, and the prediction errors were less than 20%.
Conclusions
This study provides a sensitive method to identify diazepam ingestion by monitoring diazepam and its metabolites including glucuronides, as well as to infer the time following oral consumption.

Similar content being viewed by others
References
Woods JH, Katz JL, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacol Rev. 1992;44:151–347.
Martin IL. The benzodiazepines and their receptors: 25 years of progress. Neuropharmacology. 1987;26:957–70.
Summa JD. Hypnotics and analgesics as a means of suicide. Munchener medizinische Wochenschrift. 1950;1966(108):2501.
Fangmin Li EA. Investigation of medicine and driving of people with mental disorders. J Clin Psychiatry. 2016;01:51.
Montgomery MA. The use of benzodiazepines to facilitate sexual assault. Forensic Sci Rev. 2010;22:33–40.
Shbair MKS, Eljabour S, Lhermitte M. Drugs involved in drug-facilitated crimes: part I: alcohol, sedative-hypnotic drugs, gamma-hydroxybutyrate and ketamine. A review. Ann Pharm Francaises. 2010;68:275.
Qiang Li EA. Introduction to international and internal influence of drug-driving. Chin Pharm J. 2013;10:759–62.
Costa E. The benzodiazepines: from molecular biology to clinical practice. New York: Raven; 1983.
Dinis-Oliveira RJ. Metabolic profile of oxazepam and related benzodiazepines: clinical and forensic aspects. Drug Metab Rev. 2017;49:451–63.
Liu D. Analysis of diazepam and its main metabolites in urine by GC-ECD. Chin J Forensic Med. 2003;18:276–8.
Vogliardi S, Favretto D, Tucci M, Stocchero G, Ferrara SD. Simultaneous LC-HRMS determination of 28 benzodiazepines and metabolites in hair. Anal Bioanal Chem. 2011;400:51–67.
Larsen HS, Chin PK, Begg EJ, Jensen BP. Quantification of total and unbound concentrations of lorazepam, oxazepam and temazepam in human plasma by ultrafiltration and LC-MS/MS. Bioanalysis. 2011;3:843–52.
Han S, Jia S, Guo L. Flow-injection chemiluminescence determination of diazepam by oxidation with N-bromosuccinimide. Luminescence. 2013;28:888–93.
Guo Y, Guo S, Zheng L, Zhang S, Zhang Y, Wang R. HPLC assay of diazepam and its metabolites in urine by hydrolysis with β-glucuronidase. Chin J Clin Pharm. 2001;10:302–4.
Fu S, Lewis J, Wang H, Keegan J, Dawson M. A novel reductive transformation of oxazepam to nordiazepam observed during enzymatic hydrolysis. J Anal Toxicol. 2010;34:243–51.
Fu S, Molnar A, Bowron P, Lewis J, Wang H. Reduction of temazepam to diazepam and lorazepam to delorazepam during enzymatic hydrolysis. Anal Bioanal Chem. 2011;400:153–64.
Wang X, Wang R, Zhang Y, Liang C, Ye H, Cao F, Rao Y. Extending the detection window of diazepam by directly analyzing its glucuronide metabolites in human urine using liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1268:29–34.
Rong W, Xin W, Chen L, Ni C, Xiong L, Rao Y, Zhang Y. Direct determination of diazepam and its glucuronide metabolites in human whole blood by μElution solid-phase extraction and liquid chromatography–tandem mass spectrometry. Forensic Sci Int. 2013;233:304–11.
Linchuan Liao YW. Forensic toxicology analysis. Beijing: People’s Medical; 2009.
He W. Study on the Dynamic Distribution and Postmortem Diffusion of Diazepam and its Metabolites in Rabbits: Shanxi Medical University, 2013.
Bogusz MJ, Maier RD, Driessen S. Morphine, morphine-3-glucuronide, morphine-6-glucuronide, and 6-monoacetylmorphine determined by means of atmospheric pressure chemical ionization-mass spectrometry-liquid chromatography in body fluids of heroin victims. J Anal Toxicol. 1997;21:346–55.
Friedman H, Greenblatt DJ. Pharmacokinetics and pharmacodynamics of oral diazepam: effect of dose, plasma concentration, and time. Clin Pharmacol Ther. 1992;52:139–50.
David J, Greenblatt JSHF. A large-sample study of diazepam pharmacokinetics. Ther Drug Monit. 1989;11:652–7.
Jones AWPD, Larsson HB. Distribution of diazepam and nordiazepam between plasma and whole blood and the influence of hematocrit. Ther Drug Monit. 2004;26:380–5.
Marcucci F, Guaitani A, Kvetina J, Mussini E, Garattini S. Species difference in diazepam metabolism and anticonvulsant effect. Eur J Pharmacol. 1968;4:467–70.
Bertilsson L, Baillie TA, Reviriego J. Factors influencing the metabolism of diazepam. Pharmacol Ther. 1990;45:85–91.
Zhang Y, Reviriego J, Lou YQ, Sjöqvist F, Bertilsson L. Diazepam metabolism in native Chinese poor and extensive hydroxylators of S-mephenytoin: interethnic differences in comparison with white subjects. Clin Pharmacol Ther. 1990;48:496–502.
Jones AW, Holmgren A, Holmgren P. High concentrations of diazepam and nordiazepam in blood of impaired drivers: association with age, gender and spectrum of other drugs present. Forensic Sci Int. 2004;146:1–07.
Gershkovich P, Wasan KM, Ribeyre C, Ibrahim F, Mcneill JH. Effect of variations in treatment regime and liver cirrhosis on exposure to benzodiazepines during treatment of alcohol withdrawal syndrome. Drugs Context. 2015;4:212287.
Qiang Yang EA. Study on the pharmacokinetics of diazepam. Chin Bull Drug Depend. 1993;02:97.
Emilio Perucca MPGG, Edoardo Spina MPSB, Marina Gips MAMB. Inhibition of diazepam metabolism by fluvoxamine—a pharmacokinetic study in normal volunteers. Clin Pharmacol Ther. 1994;56:471–6.
Özdemir M, Aktan Y, Boydaĝ BS, Cingi MI, Musmul A. Interaction between grapefruit juice and diazepam in humans. Eur J Drug Metab Pharmacokinet. 1998;23:55–9.
Kosuge K, Jun Y, Watanabe H, Kimura M, Nishimoto M, Ishizaki T, Ohashi K. Effects of CYP3A4 inhibition by diltiazem on pharmacokinetics and dynamics of diazepam in relation to CYP2C19 genotype status. Drug Metab Dispos Biol Fate Chem. 2001;29:1284.
Sakai N, Ishizuka M. Impact of rat P450 genetic polymorphism on diazepam metabolism. Expert Opin Drug Metab. 2009;5:1421–33.
Peng J, Liu W. Research progress in the effect of CYP2C19 gene polymorphisms on drug metabolism. J Pharm Pract. 2015;33:508–12.
Qin X. Difference of Metabolism of Diazepam Between Two Groups of Genotype. J Changzhi Med Coll. 1999;13:166–8.
Acknowledgements
We thank Shanlin Fu from University of Technology Sydney, Australia and Meng Hu from ShanXi Medical University, China for their help in the language and editing of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
The study was funded by the NSFC (81172906), National Key Technology R & D Program of China (2007BAK26B05 and 2012BAK02B02) and National Science and Technology Special Project Work (SQ2015FYJ010051).
Conflict of interest
The authors declared that they had no conflicts of interest to this work.
Ethics approval
The study was approved by the Committee of Medical Ethics of Shanxi Medical University (2014087). All methods were performed in accordance with the relevant guidelines and regulations in China concerning the use of human subjects in scientific research.
Informed consent
All subjects provided written informed consent prior to screening.
Rights and permissions
About this article
Cite this article
Wang, Ll., Ren, Xx., He, Y. et al. Study on the Pharmacokinetics of Diazepam and Its Metabolites in Blood of Chinese People. Eur J Drug Metab Pharmacokinet 45, 477–485 (2020). https://doi.org/10.1007/s13318-020-00614-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-020-00614-8