Abstract
Introduction
Dementia is quite prevalent and among the leading causes of death worldwide. According to earlier research, diabetes may increase the possibility of developing dementia. However, the association between antidiabetic agents and dementia is not yet clear. This investigation examines the association between the use of sodium-glucose transporter 2 inhibitors (SGLT2i) and the risk of dementia in patients with diabetes.
Methods
Up to April 18, 2023, four databases—Europe PMC, Medline, Scopus, and Cochrane Library—were searched for relevant literature. We included all studies that examine dementia risk in adults with diabetes who use SGLT2i. Random-effect models were used to compute the outcomes in this investigation, producing pooled odds ratios (OR) with 95% confidence intervals (CI).
Results
Pooled data from seven observational studies revealed that SGLT2i use was linked to a lower risk of dementia in people with diabetes (OR 0.45, 95% CI 0.34–0.61; p < 0.00001, I2 = 97%). The reduction in the risk of dementia due to SGLT2i’s neuroprotective effect was only significantly affected by dyslipidemia (p = 0.0004), but not by sample size (p = 0.2954), study duration (p = 0.0908), age (p = 0.0805), sex (p = 0.5058), hypertension (p = 0.0609), cardiovascular disease (p = 0.1619), or stroke (p = 0.2734).
Conclusions
According to this research, taking SGLT2i reduces the incidence of dementia in people with diabetes by having a beneficial neuroprotective impact. Randomized controlled trials (RCTs) are still required in order to verify the findings of our research.
Similar content being viewed by others
Why carry out this study? |
Currently the association between antidiabetic agents, especially sodium glucose transporter 2 inhibitors (SGLT2i), and dementia is still unclear. |
This study aims to analyze the association between SGLT2i use and the incidence of dementia in patients with diabetes. |
What was learned from the study? |
Our pooled analysis from seven observational studies showed that the incidence of dementia was significantly lower in patients with diabetes who used SGLT2i as their glucose-lowering medications. |
Further regression analysis showed that the association between SGLT2i and the risk of dementia was significantly influenced by dyslipidemia. |
Our study suggests that SGLT2i may offer beneficial effects in reducing dementia incidence in patients with diabetes. |
Introduction
The syndrome known as dementia is characterized by disorientation of memory, thought processes, behavior, and decreased ability to carry out daily activities [1, 2]. This syndrome is chronic and progressive with the majority of dementia cases caused by Alzheimer’s disease [1, 2]. Apart from Alzheimer’s disease, other common etiologies of dementia are vascular causes, Lewy body disease, and frontotemporal lobe atrophy [2]. More than 55 million individuals worldwide suffer from dementia at the time of right writing, with more than 60% of them residing in low- to middle-income nations [3]. It is anticipated that this number will steadily rise to 152 million cases in 2050 [3]. Apart from having a fairly high prevalence, dementia is one of the main causes of disability in older individuals as well as the seventh largest cause of mortality, causing this syndrome to pose a high disease burden and affect the quality of life [4].
Numerous factors, including advanced age, high blood pressure, diabetes, obesity, smoking, and alcohol use, increase the likelihood of developing dementia [5]. Besides those factors, recent meta-analyses have also discovered that diabetes mellitus (DM) may be linked to a higher risk of dementia [6, 7]. It has been hypothesized that there are overlapping risk factors between DM and dementia, such as age, obesity, hyperlipidemia, and hypertension which are the main risk factors for both DM and dementia [7, 8]. Diabetes also increases the risk of developing cerebrovascular disease which is closely related to the occurrence of dementia, especially vascular dementia [7, 8]. In addition, the hyperglycemia that occurs in diabetes is often associated with the accelerated formation of advanced glycated end products (AGE) [7, 8]. Cross-linking of extracellular protein mediated by AGE will accelerate the aggregation of β-amyloid, the main protein in the pathogenesis of Alzheimer’s dementia [7, 8]. Finally, insulin receptors in the cerebral cortex and hippocampus play an important role in memory function [7, 8]. Insulin will cause the release of the β-amyloid peptide to the outer layer of the cell and encourage the development of the enzyme that breaks down the peptide, known as the insulin-degrading enzyme (IDE) [7, 8]. Insulin resistance will thereby encourage the buildup of β-amyloid, increasing the risk of Alzheimer’s disease [7, 8]. The next question that arises is whether antidiabetic drugs can also affect the prevalence of dementia in people with diabetes. Since the US Food and Drug Administration (FDA) initially authorized sodium-glucose transporter 2 inhibitors (SGLT2i) in 2013 as antidiabetic drugs, they have been extensively researched and are able to provide benefits in various conditions, such as lowering the likelihood of cardiovascular hospitalization and death among patients with heart failure [9], along with lowering the death rates in people with chronic kidney disease (CKD) and delaying renal disease progression [10]. Unfortunately, the existing literature regarding the effect of SGLT2i on the risk of dementia is still scarce and unclear. In this research, we sought to examine the relationship between SGLT2i usage and dementia incidence in populations with diabetes.
Methods
Eligibility Criteria
The present study was conducted following the Meta-analysis of Observational Studies in Epidemiology (MOOSE) [11] and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12]. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The Faculty of Medicine, Pelita Harapan University Research Ethics Committee confirmed that no ethical approval was required. The protocol of this review has been registered in PROSPERO (CRD42023420793). The inclusion criteria for this review were set using the PECOS formulation, and studies were considered if they met these criteria: (1) Population type: patients with DM who had no history of dementia at the start of the study; (2) Exposure type: utilizing SGLT2i as a treatment for lowering their blood sugar; (3) Control type: has no history of SGLT2i use but used other antidiabetic agents besides SGLT2i; (4) Outcome type: incidence of dementia. Dementia in this study is defined as a general term for loss of memory, language, problem-solving, and other thinking abilities that are severe enough to interfere with daily life. Dementia analyzed can be in the form of Alzheimer’s dementia, vascular dementia, Lewy body dementia, or frontotemporal dementia; (5) Study design type: clinical trials and observational research. Studies were excluded from this review if one or more of the following exclusion criteria were present: (1) studies in a population of patients who already had dementia without diabetes; (2) studies assessing the effect of antidiabetic agents on the disease course and outcome of dementia; (3) scholarly works that are not available as full text; (4) case-series and case-reports; (5) non-primary research.
Search Strategy and Study Selection
A query with the search term consisting of words related to “diabetes”, “sodium glucose transporter 2 inhibitors”, and “dementia” was searched through four databases—Europe PMC, Scopus, Medline, and Cochrane Library—for papers published in English up to April 18, 2023. The search strategy employed in each database was described in detail in Supplementary Table 1. Initially, two reviewers conducted a selection of articles based on titles and abstracts. All duplicates were eliminated. The full text articles after abstract screening were downloaded and verified for inclusion in the study and any disagreement was resolved by asking for opinions from a third, independent reviewer.
Data Extraction
Data extraction was performed by two separate authors to collect important information from included studies as follows: name of the authors, country of origin, year of publication, design of the research, sample size, timeframe of the study, and characteristics of study participants (mean age, sex, hypertension, dyslipidemia, cardiovascular disease, and stroke), the numbers of subjects in the experimental group and comparison group, and the outcome of interest in each group. Any discrepancy between authors at this stage was resolved through discussion.
Risk of Bias Assessment
Using a suitable and verified tool, two independent reviewers evaluated the included studies’ risk of bias. The included observational studies’ quality was evaluated using the Newcastle–Ottawa scale (NOS), which has three rating criteria: the process of choosing study participants; similarity between each group’s participants; and the findings in each study [13]. Each study might receive a total score ranging from 0 to 9, with a score of 7 indicating good quality research [13].
Statistical Analysis
The Mantel–Haenszel calculation was employed to determine the incidence of dementia, which served as the primary outcome in this investigation. The results were presented in the form of an odds ratio (OR) accompanied by a 95% confidence interval (95% CI). Dementia in this study was defined according to the International Classification of Diseases 10th Revision (ICD-10) with following codes: F00, F01, F02, F03, G30, and G31. Because of the various participant characteristics, different design of the study, and variable length of the investigation, heterogeneity was anticipated and the random-effect model was adopted in this study. The I-squared (I2) statistics were employed to assess the heterogeneity among studies, where values exceeding 50% indicated a substantial or noteworthy level of heterogeneity [14]. In order to assess the potential interaction effect between the use of SGLT2 inhibitors and pre-defined variables including sample size, study duration, age, gender, hypertension, dyslipidemia, cardiovascular disease, and stroke, a meta-regression analysis was conducted. This analysis employed a random-effects model and utilized a restricted-maximum likelihood approach. In instances where the number of papers included in a meta-analysis exceeds 10, a funnel plot was employed as a means to evaluate the presence of publication bias. The software tools employed for conducting meta-analyses in this study include the Cochrane Collaboration’s Review Manager 5.4 and Comprehensive Meta-Analysis.
Results
Study Selection and Characteristics
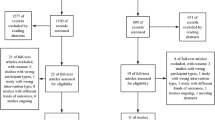
The process of conducting literature searches across four databases yielded a cumulative total of 451 studies. A total of 429 studies were excluded on the basis screening the titles/abstracts and removing duplicates, leaving 22 studies for full-text assessment. Fifteen of these studies were further excluded for the following reasons: seven studies were review articles, five studies were not done in human subjects, and three studies lacked data on the pre-specified outcomes. Ultimately, seven studies [15,16,17,18,19,20,21] were included in the review which encompassed a total of 627,275 patients with DM (Fig. 1). Three of these studies were case–control studies, two were prospective cohort studies, and two were retrospective cohort studies. All of the included studies came from different countries, namely Japan, China, Taiwan, Germany, Sweden, Denmark, and Canada. The duration of the studies ranged from 4 to 17 years. All of the included studies assessed the incidence of dementia as their primary outcome. Dementia type evaluated by the included studies encompassed a wide variety, starting from dementia in Alzheimer’s disease, vascular dementia, dementia with Lewy body, frontotemporal dementia, and dementia in Parkinson’s disease. Table 1 provides further information pertaining to the fundamental characteristics of the studies that were incorporated.
Quality of Study Assessment
According to the risk of bias evaluation utilizing the NOS tool, all included studies were of good quality, so they were considered worthy of being included in the pooled analysis (Table 2).
Incidence of Dementia (Crude Analysis)
Our pooled analysis of seven observational studies revealed that, in comparison to other antidiabetic drugs, the use of SGLT2i in individuals with DM reduced their chance of acquiring dementia (OR 0.45, 95% CI 0.34–0.61; p < 0.00001, I2 = 97%, random-effect model) (Fig. 2).
Meta-Regression
Through the use of meta-regression, risk factors that can affect the association between SGLT2i use and the incidence of dementia were identified (Supplementary Table 2). Our regression analysis showed that the sole factor specifically influencing the risk of dementia in patients with DM who used SGLT2i was dyslipidemia (beta coefficient 0.0211; 95% CI 0.0095–0.0327; p = 0.0004) (Supplementary Fig. 1A) where higher dyslipidemia prevalence will increase the incidence of dementia. Meanwhile other factors such as sample size (p = 0.2954) (Supplementary Fig. 1B), study duration (p = 0.0908) (Supplementary Fig. 1C), age (p = 0.0805) (Supplementary Fig. 1D), sex (p = 0.5058) (Supplementary Fig. 1E), hypertension (p = 0.0609) (Supplementary Fig. 1F), cardiovascular disease (p = 0.1619) (Supplementary Fig. 1G), and stroke (p = 0.2734) (Supplementary Fig. 1H) did not significantly influenced the relationship between SGLT2i usage and dementia risk.
Publication Bias
As a result of the limited number of publications (fewer than 10) available for each outcome in this review, the reliability of funnel plots and statistical tests for detecting publication bias is diminished [22, 23]. Consequently, the present study did not undertake an examination of publication bias.
Discussion
On the basis of our comprehensive meta-analysis of seven observational studies, it has been determined that the utilization of SGLT2i as therapeutic agents for DM can effectively reduce the occurrence of dementia. Additional regression analysis also revealed that the sole factor significantly influencing this relationship was dyslipidemia. However, other factors such as sample size, duration of the study, age, sex, hypertension, cardiovascular disease, or stroke did not significantly influence this relationship.
Several hypotheses might explain why the use of SGLT2i may lower the risk of dementia. First, previous studies have indicated that cognitive impairment, including dementia, is strongly associated with the presence of atherosclerotic lesions in the intracranial and extracranial arteries [24,25,26]. For example, the study by Dearborn et al. [24] demonstrated that the prevalence of dementia in older adults was shown to be higher when atherosclerotic plaques were found in the anterior cerebral artery (RPR 3.81 95% CI [1.57–9.23] p = 0.003). Another study by Wingo et al. [26] demonstrated that independent of ischemia, cerebral atherosclerosis was linked to impaired synapse function, increased myelination, and axonal damage. Therefore, preventing the occurrence of cerebral atherosclerosis will help improve cognitive function in older adults [24,25,26]. SGLT2i has pleiotropic properties through its effects on reducing blood vessel inflammation, and oxidative stress, and repairing endothelial dysfunction so that it can prevent atherosclerosis [27]. This argument is further supported by research by Irace et al. [28] which showed that therapy with empagliflozin for 3 months was able to reduce a marker of early atherosclerosis, namely complex intima-media thickness (cMIT) by 7.9% (p < 0.0001) in patients with diabetes. Additionally, people with type 2 diabetes mellitus who have cMIT have diminished cognitive performance, so the reduction of cMIT through SGLT2i therapy will have an impact on preventing the deterioration of cognitive function, including dementia [29]. Since one of the major risk factors for developing atherosclerosis is dyslipidemia, the above statements may also explain the results from our meta-regression analysis which indicate that the risk of developing dementia in patients with DM who used SGLT2i was significantly influenced by dyslipidemia.
Second, available evidence also suggests that dementia and cognitive impairment may both be influenced by systemic inflammation outside the central nervous system (CNS) [30]. Walker et al. [31] found that after 20 years, there is a significant deterioration in cognitive function due to higher inflammatory markers during middle adulthood. The neurovascular unit (NVU), which is made up of neurons, microglia, astrocytes, and pericytes as well as endothelial cells lining the brain microvessels, regulates homeostasis by controlling blood flow to the neuronal environment [32]. Circulation of proinflammatory cytokines, which have been associated with systemic inflammation, has been found to impair the permeability of the blood–brain barrier, induce a proinflammatory phenotype in astrocytes and microglia, and cause damage to the endothelium of brain microvessels [32]. M1-activated microglia will interfere with the NVU by producing proinflammatory cytokines such as tumor necrosis factor alpha (TNFα), interleukin (IL)-1β, IL-6, and IL-18 which will stimulate tau hyperphosphorylation, oligomerization of β-amyloid, complement activation, and break down neurotransmitters into bioactive metabolites causing neurodegeneration [33, 34]. In addition, the development of atherosclerotic plaques is aided by this proinflammatory cytokine [34, 35]. The administration of SGLT2 inhibitors impedes the remodeling of several components of the neurovascular unit, including the blood–brain barrier, pericytes, astrocytes, microglia, and oligodendrocytes [34, 36]. Consequently, this inhibitory effect serves to limit the progression of neurodegenerative processes [34, 36]. SGLT2i is also able to reduce the expression of proinflammatory cytokines thereby inhibiting the progression of atherosclerosis [27, 34].
Third, the activation of NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) inflammasome is an important factor in the pathogenesis of atherosclerosis and Alzheimer’s disease, two things that are closely related to dementia [37]. Activation of the NLRP3 pathway in the arterial wall by lipoproteins will trigger an inflammatory process that encourages the formation of atherosclerotic plaques [38]. The NLRP3 inflammasome can also trigger neuroinflammation and interfere with the removal of amyloid-β by microglia, thereby increasing the risk of Alzheimer’s disease [39]. SGLT2i is able to inhibit the NLRP3 inflammasome thereby improving atherosclerosis and preventing cognitive dysfunction [34, 40].
Fourth, the occurrence of dementia, specifically Alzheimer’s dementia and vascular dementia, is additionally linked to the existence of oxidative damage resulting from the generation of reactive oxygen species (ROS) during the chronic inflammatory process [41]. This oxidative stress will stimulate the aggregation, phosphorylation, and polymerization of amyloid-β (Aβ) and tau peptides in the CNS thereby causing a process of neurodegeneration in which there are a loss of neurons and synapses, disturbances in synaptic plasticity, disturbances in neurotransmitter balance, and neuroinflammation that subsequently result in cognitive decline, including dementia [42]. SGLT2i have been observed to mitigate oxidative stress in individuals diagnosed with DM. This is achieved by the suppression of free radical generation and the promotion of antioxidative enzyme activity, including glutathione S-reductase and catalase [43].
Finally, what is no less important in the pathogenesis of dementia, especially Alzheimer’s dementia, is mTOR (mechanistic/mammalian target of rapamycin) signaling which links metabolic disease with cognitive disorders [44]. In patients with DM, there is a dysregulation of mTOR signaling in which not only atherosclerosis happens as a result of endothelial cell dysfunction but also hyperphosphorylation and aggregation of Aβ and tau peptides which play a role in the pathogenesis of Alzheimer’s disease [45]. SGLT2i is able to suppress mTOR signaling by shifting metabolism from an anabolic to a catabolic state through the activation of glycogenolysis and gluconeogenesis which occurs particularly at night [46, 47]. The transition from an anabolic to a catabolic state is not contingent upon glucose and insulin, but rather relies on the process of fatty acid oxidation, which subsequently decreases the availability of mTOR fuel, specifically blood insulin and amino acids [46, 47]. With this suppression of mTOR signaling, the process of dementia, especially Alzheimer’s dementia, can also be inhibited [46, 47].
Previous studies have also explored the relationship between other antidiabetic agents, such as metformin and glucagon-like peptide 1 (GLP-1) receptor agonists, and the dementia risk [48,49,50]. A meta-analysis published in 2018 that pooled data from 14 studies showed that the use of metformin was associated with less cognitive impairment in patients with diabetes [48]. This meta-analysis also showed that the incidence of dementia was significantly reduced among patients with diabetes who used metformin when compared to control group [48]. Meanwhile, regarding GLP-1 receptor agonist, a previous meta-analysis revealed that the use of GLP-1 receptor agonist can significantly improve cognitive function in patients with Alzheimer’s disease [49]. This beneficial effect from GLP-1 receptor agonist on cognitive function was further confirmed by another recent meta-analysis study that showed a lower risk of all-cause dementia with the use of GLP-1 receptor agonist [50]. All of this information has provided us with evidence that antidiabetic agents may have a role in altering the development of dementia in patients with diabetes.
Our research has some limitations. First, the evidence generated from our research only comes from observational studies with different study design (prospective cohort, retrospective cohort, and case–control) which are very likely to be influenced by confounding factors as well as some biases, such as recall bias and information bias, and therefore must be interpreted with caution. However, we have tried our best to minimize the presence of confounding factors by conducting a meta-regression analysis. Second, there is significant heterogeneity in the outcome of interest in this study, which is very likely due to differences in the study duration and differences in the baseline characteristics of the studies’ participants, such as differences in the duration of SGLT2i. The information regarding the duration of diabetes, type, dose, and duration of SGLT2i use in the included studies is lacking and therefore cannot be analyzed further. Moreover, all of the included studies only provided the information regarding the effect of SGLT2i use on the overall dementia incidence without providing separate information regarding the effect of SGLT2i use on each type of dementia. The absence of this information hinders our ability to synthesize evidence for the specific type of dementia that would derive the greatest advantage from the utilization of SGLT2i. Finally, most of the studies included in the analysis had a relatively small follow-up period (less than 10 years), which posed a challenge in thoroughly evaluating cognitive impairment (dementia) that necessitates long-term observation. However, we believe that the results of our research can still have implications for clinical practice. Future research should be in the form of randomized controlled trials (RCTs) with long duration of follow-up and include complete data on the type, dose, and duration of SGLT2i usage to analyze the influence of these variables on the incidence of dementia among patients with DM.
Conclusion
Our study, a systematic review and meta-analysis, has shown that the utilization of SGLT2i in individuals diagnosed with DM exhibits a promising beneficial effect in mitigating the likelihood of developing dementia. The observed positive impact of SGLT2i is found to be significantly influenced by dyslipidemia, while other parameters such as age, sex, and comorbid conditions do not exhibit a significant influence on this effect. Nonetheless, it is still imperative to conduct suitable and meticulously planned RCTs in order to validate the findings of our study.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
References
Hariyanto TI, Putri C, Arisa J, Situmeang RFV, Kurniawan A. Dementia and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review and meta-analysis. Arch Gerontol Geriatr. 2021;93:104299. https://doi.org/10.1016/j.archger.2020.104299.
Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and management of dementia: review. JAMA. 2019;322(16):1589–99. https://doi.org/10.1001/jama.2019.4782.
GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105–e125. https://doi.org/10.1016/S2468-2667(21)00249-8.
GBD 2019 Collaborators. Global mortality from dementia: application of a new method and results from the Global Burden of Disease Study 2019. Alzheimers Dement (N Y). 2021;7(1):e12200. https://doi.org/10.1002/trc2.12200.
Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46. https://doi.org/10.1016/S0140-6736(20)30367-6.
Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42(5):484–91. https://doi.org/10.1111/j.1445-5994.2012.02758.x.
Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J Diabetes Invest. 2013;4(6):640–50. https://doi.org/10.1111/jdi.12087.
Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14(10):591–604. https://doi.org/10.1038/s41574-018-0048-7.
Tsampasian V, Baral R, Chattopadhyay R, et al. The role of SGLT2 inhibitors in heart failure: a systematic review and meta-analysis. Cardiol Res Pract. 2021;19(2021):9927533. https://doi.org/10.1155/2021/9927533.
Bailey CJ, Day C, Bellary S. Renal protection with SGLT2 inhibitors: effects in acute and chronic kidney disease. Curr Diab Rep. 2022;22(1):39–52. https://doi.org/10.1007/s11892-021-01442-z.
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. https://doi.org/10.1001/jama.283.15.2008.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;29(372): n71. https://doi.org/10.1136/bmj.n71.
Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 20 Apr 2023.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557.
Akimoto H, Negishi A, Oshima S, et al. Antidiabetic drugs for the risk of Alzheimer disease in patients with type 2 DM using FAERS. Am J Alzheimers Dis Other Dement. 2020;35:1533317519899546. https://doi.org/10.1177/1533317519899546.
Bohlken J, Jacob L, Kostev K. Association between the use of antihyperglycemic drugs and dementia risk: a case–control study. J Alzheimers Dis. 2018;66(2):725–32. https://doi.org/10.3233/JAD-180808.
Mui JV, Zhou J, Lee S, et al. Sodium-glucose cotransporter 2 (SGLT2) inhibitors vs dipeptidyl peptidase-4 (DPP4) inhibitors for new-onset dementia: a propensity score-matched population-based study with competing risk analysis. Front Cardiovasc Med. 2021;8:747620. https://doi.org/10.3389/fcvm.2021.747620.
Secnik J, Xu H, Schwertner E, et al. Dementia diagnosis is associated with changes in antidiabetic drug prescription: an open-cohort study of ∼130,000 Swedish subjects over 14 years. J Alzheimers Dis. 2020;76(4):1581–94. https://doi.org/10.3233/JAD-200618.
Siao WZ, Lin TK, Huang JY, Tsai CF, Jong GP. The association between sodium-glucose cotransporter 2 inhibitors and incident dementia: a nationwide population-based longitudinal cohort study. Diab Vasc Dis Res. 2022;19(3):14791641221098168. https://doi.org/10.1177/14791641221098168.
Wium-Andersen IK, Osler M, Jørgensen MB, Rungby J, Wium-Andersen MK. Antidiabetic medication and risk of dementia in patients with type 2 diabetes: a nested case-control study. Eur J Endocrinol. 2019;181(5):499–507. https://doi.org/10.1530/EJE-19-0259.
Wu CY, Iskander C, Wang C, et al. Association of sodium-glucose cotransporter 2 inhibitors with time to dementia: a population-based cohort study. Diabetes Care. 2023;46(2):297–304. https://doi.org/10.2337/dc22-1705.
Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol. 2000;53(2):207–16. https://doi.org/10.1016/s0895-4356(99)00161-4.
Terrin N, Schmid CH, Lau J, Olkin I. Adjusting for publication bias in the presence of heterogeneity. Stat Med. 2003;22(13):2113–26. https://doi.org/10.1002/sim.1461.
Dearborn JL, Zhang Y, Qiao Y, et al. Intracranial atherosclerosis and dementia: the Atherosclerosis Risk in Communities (ARIC) Study. Neurology. 2017;88(16):1556–63. https://doi.org/10.1212/WNL.0000000000003837.
Bos D, Vernooij MW, de Bruijn RF, et al. Atherosclerotic calcification is related to a higher risk of dementia and cognitive decline. Alzheimers Dement. 2015;11(6):639-47.e1. https://doi.org/10.1016/j.jalz.2014.05.1758.
Wingo AP, Fan W, Duong DM, et al. Shared proteomic effects of cerebral atherosclerosis and Alzheimer’s disease on the human brain. Nat Neurosci. 2020;23(6):696–700. https://doi.org/10.1038/s41593-020-0635-5.
Liu Z, Ma X, Ilyas I, et al. Impact of sodium glucose cotransporter 2 (SGLT2) inhibitors on atherosclerosis: from pharmacology to pre-clinical and clinical therapeutics. Theranostics. 2021;11(9):4502–15. https://doi.org/10.7150/thno.54498.
Irace C, Casciaro F, Scavelli FB, et al. Empagliflozin influences blood viscosity and wall shear stress in subjects with type 2 diabetes mellitus compared with incretin-based therapy. Cardiovasc Diabetol. 2018;17(1):52. https://doi.org/10.1186/s12933-018-0695-y.
Feinkohl I, Keller M, Robertson CM, et al. Clinical and subclinical macrovascular disease as predictors of cognitive decline in older patients with type 2 diabetes: the Edinburgh type 2 diabetes study. Diabetes Care. 2013;36(9):2779–86. https://doi.org/10.2337/dc12-2241.
Walker KA, Ficek BN, Westbrook R. Understanding the role of systemic inflammation in Alzheimer’s disease. ACS Chem Neurosci. 2019;10(8):3340–2. https://doi.org/10.1021/acschemneuro.9b00333.
Walker KA, Gottesman RF, Wu A, et al. Systemic inflammation during midlife and cognitive change over 20 years: the ARIC study. Neurology. 2019;92(11):e1256–67. https://doi.org/10.1212/WNL.0000000000007094.
Rochfort KD, Cummins PM. The blood-brain barrier endothelium: a target for pro-inflammatory cytokines. Biochem Soc Trans. 2015;43(4):702–6. https://doi.org/10.1042/BST20140319.
Wang WY, Tan MS, Yu JT, Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. 2015;3(10):136. https://doi.org/10.3978/j.issn.2305-5839.2015.03.49.
Pawlos A, Broncel M, Woźniak E, Gorzelak-Pabiś P. Neuroprotective effect of SGLT2 inhibitors. Molecules. 2021;26(23):7213. https://doi.org/10.3390/molecules26237213.
Fatkhullina AR, Peshkova IO, Koltsova EK. The role of cytokines in the development of atherosclerosis. Biochemistry (Mosc). 2016;81(11):1358–70. https://doi.org/10.1134/S0006297916110134.
Hayden MR, Grant DG, Aroor AR, DeMarco VG. Empagliflozin ameliorates type 2 diabetes-induced ultrastructural remodeling of the neurovascular unit and neuroglia in the female db/db mouse. Brain Sci. 2019;9(3):57. https://doi.org/10.3390/brainsci9030057.
Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328. https://doi.org/10.3390/ijms20133328.
Jin Y, Fu J. Novel insights into the NLRP 3 inflammasome in atherosclerosis. J Am Heart Assoc. 2019;8(12):e012219. https://doi.org/10.1161/JAHA.119.012219.
Tejera D, Mercan D, Sanchez-Caro JM, et al. Systemic inflammation impairs microglial Aβ clearance through NLRP3 inflammasome. EMBO J. 2019;38(17):e101064. https://doi.org/10.15252/embj.2018101064.
Kim SR, Lee SG, Kim SH, et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020;11(1):2127. https://doi.org/10.1038/s41467-020-15983-6.
Luca M, Luca A, Calandra C. The role of oxidative damage in the pathogenesis and progression of Alzheimer’s disease and vascular dementia. Oxid Med Cell Longev. 2015;2015:504678. https://doi.org/10.1155/2015/504678.
Huang WJ, Zhang X, Chen WW. Role of oxidative stress in Alzheimer’s disease. Biomed Rep. 2016;4(5):519–22. https://doi.org/10.3892/br.2016.630.
Yaribeygi H, Atkin SL, Butler AE, Sahebkar A. Sodium-glucose cotransporter inhibitors and oxidative stress: an update. J Cell Physiol. 2019;234(4):3231–7. https://doi.org/10.1002/jcp.26760.
Mao Z, Zhang W. Role of mTOR in glucose and lipid metabolism. Int J Mol Sci. 2018;19(7):2043. https://doi.org/10.3390/ijms19072043.
Van Skike CE, Galvan V. A perfect sTORm: the role of the mammalian target of rapamycin (mTOR) in cerebrovascular dysfunction of Alzheimer’s disease: a mini-review. Gerontology. 2018;64(3):205–11. https://doi.org/10.1159/000485381.
Stanciu GD, Rusu RN, Bild V, Filipiuc LE, Tamba BI, Ababei DC. Systemic actions of SGLT2 inhibition on chronic mTOR activation as a shared pathogenic mechanism between Alzheimer’s disease and diabetes. Biomedicines. 2021;9(5):576. https://doi.org/10.3390/biomedicines9050576.
Packer M. SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care. 2020;43(3):508–11. https://doi.org/10.2337/dci19-0074.
Campbell JM, Stephenson MD, de Courten B, Chapman I, Bellman SM, Aromataris E. Metformin use associated with reduced risk of dementia in patients with diabetes: a systematic review and meta-analysis. J Alzheimers Dis. 2018;65(4):1225–36. https://doi.org/10.3233/JAD-180263.
Bi Z, Wang L, Wang W. Evaluating the effects of glucagon-like peptide-1 receptor agonists on cognitive function in Alzheimer's disease: a systematic review and meta-analysis. Adv Clin Exp Med. 2023;32(11):1223–1231. https://doi.org/10.17219/acem/161734.
Tang H, Shao H, Shaaban CE, et al. Newer glucose-lowering drugs and risk of dementia: a systematic review and meta-analysis of observational studies. J Am Geriatr Soc. 2023;71(7):2096–106. https://doi.org/10.1111/jgs.18306.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Conceptualization: Pricilla Yani Gunawan, Paskalis Andrew Gunawan, Timotius Ivan Hariyanto; Methodology: Timotius Ivan Hariyanto; Formal analysis and investigation: Pricilla Yani Gunawan, Paskalis Andrew Gunawan, Timotius Ivan Hariyanto; Writing—original draft preparation: Timotius Ivan Hariyanto; Writing—review and editing: Pricilla Yani Gunawan, Paskalis Andrew Gunawan; Funding acquisition: Pricilla Yani Gunawan, Paskalis Andrew Gunawan; Resources: Pricilla Yani Gunawan, Paskalis Andrew Gunawan, Timotius Ivan Hariyanto.
Corresponding author
Ethics declarations
Conflict of Interest
The authors (Pricilla Yani Gunawan, Paskalis Andrew Gunawan and Timotius Ivan Hariyanto) report there are no competing interests to declare.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The Faculty of Medicine, Pelita Harapan University Research Ethics Committee has confirmed that no ethical approval is required.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gunawan, P.Y., Gunawan, P.A. & Hariyanto, T.I. Risk of Dementia in Patients with Diabetes Using Sodium-Glucose Transporter 2 Inhibitors (SGLT2i): A Systematic Review, Meta-Analysis, and Meta-Regression. Diabetes Ther 15, 663–675 (2024). https://doi.org/10.1007/s13300-024-01538-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-024-01538-1