Abstract
Introduction
Latent autoimmune diabetes in adults (LADA) is a highly heterogeneous autoimmune condition with clinical and genetic characteristics that fall between those of type 1 diabetes mellitus and type 2 diabetes mellitus; therefore, there are no uniform criteria for the selection of therapeutic agents. We conducted a network meta-analysis to evaluate the efficacy of various therapeutic agents for LADA by comparing their effects on various indicators used to reflect LADA.
Methods
We searched the PubMed, Cochrane Library, Embase and Web of Science databases from their inception to March 2023 and collected data from 14 randomized controlled trials on glucose-lowering drugs for LADA, including 23 studies and 15 treatment regimens. The effectiveness of drugs was ranked and evaluated by combining surface under the cumulative ranking (SUCRA) plots and forest plots. Factors that may influence study heterogeneity were also searched and analyzed by combining subgroup analysis, publication bias, funnel plots and sensitivity analysis.
Results
The results of the network meta-analysis showed that insulin had the most significant effect on the control of change from baseline in glycosylated hemoglobin, type A1 (ΔHbA1c). Insulin combined with dipeptidyl peptidase-4 (DPP-4) inhibitors performed the best in reducing fasting blood glucose and body mass index. Treatment regimens involving thiazolidinediones were the most advantageous in HbA1c, fasting C-peptide and postprandial C-peptide control. Longer dosing may be more beneficial in maintaining islet β-cell function in the LADA population.
Conclusion
LADA is an immune condition with high heterogeneity, and treatment should be administered according to the C-peptide level of the LADA population. For this population with LADA with a certain level of β-cell function, combinations of insulin with DPP-4 inhibitors or thiazolidinediones probably can be more effective treatment options to maintain islet function and normal blood glucose.
Trial Registration
PROSPERO CRD42023410795.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Although latent autoimmune diabetes in adults (LADA) has received increasing attention as an autoimmune condition affecting the widest population, there is a lack of real and strong evidence on the effectiveness of treatment options for LADA. |
This study is based on the hypothesis that no uniform drug administration strategy has been formed for the treatment of LADA. Meta-analysis studies on residual β-cell function, an indicator to assess the effectiveness of LADA treatment regimens, have not been reported in the literature. |
What was learned from this study? |
In the LADA population who have reached a certain level of β-cell function, combinations of insulin with dipeptidyl peptidase-4 inhibitors or thiazolidinediones probably can be more effective treatment options than insulin alone to maintain islet function and control blood glucose. |
Selection of treatment regimens based on C-peptide level may be beneficial to the LADA population. |
Introduction
Latent autoimmune diabetes in adults (LADA) is recognized as a part of the autoimmune diabetes spectrum and a specific form that can be characterized by a positive islet-autoantibody status and an initially insulin-independent group of subjects. Since a significant proportion of patients with LADA exhibit clinical manifestations closer to those of type 2 diabetes mellitus (T2DM), it is not uncommon for unregulated clinical practices to directly replicate T2DM treatment regimens on the LADA population [1,2,3].
In 1999, the World Health Organization (WHO) recommended that LADA be classified as a subtype of type 1 diabetes mellitus (T1DM) with a slow onset, and interventions for LADA have been controversial for several years [4]. However, LADA is a global disease with a detection rate of 10% of patients with confirmed T2DM [5,6,7]. How to select more effective therapeutic measures to preserve the function of residual islet β-cells and delay the development of complications is essential to improve the quality of life in the LADA population. Moreover, clarification of the treatment of LADA is also important for deepening the understanding of diabetes and constructing a more recognizable diabetes diagnosis and staging strategy.
LADA has a more insidious onset than T1DM [8], and its clinical features are quite similar to those of T2DM. This specificity of LADA highlights an obvious shortcoming of the current classification of diabetes, and the latest view is that severe autoimmune diabetes (SAID) is a more accurate name for the phenotype with islet-specific autoantibodies in adult-onset diabetes [2]. Because no systematic treatment guidelines have been issued for LADA, there are a variety of clinical medication measures, and no uniform drug administration strategy has been formed. Many people with LADA are still using nonautoimmune diabetes regimens, such as sulfonylureas, which accelerate the process of β-cell apoptosis and insulin dependence [9].
In addition to the current use of insulin at an early stage to protect the function of pancreatic β-cells, dipeptidyl peptidase-4 (DPP-4) inhibitors have been favored in the treatment of LADA in recent years due to their ability to enhance the biological effect of endogenous glucagon-like peptide-1 (GLP-1) and control blood glucose levels [10, 11]. Therapy involving GLP-1 receptor agonists (GLP-1RA) has been shown in a recent study to be more effective in lowering blood glucose in LADA than in T2DM [12]. A recent study has also shown that semaglutide preserves β-cell function well in the T2DM population [13, 14]. Nevertheless, clinical trials have shown that GLP-1RA therapy confers no benefit to participants with T2DM when C-peptide levels are very low or autoantibody titers are very high [15]. Therefore, there is no consensus on whether GLP-1RA therapy has a substantial effect on the course of islet impairment in the LADA population. While thiazolidinediones are able to reduce insulin dosage, delay β-cell damage and reduce the possibility of heart failure in the LADA population in recent clinical studies [16], their therapeutic potential for LADA needs to be further explored. Immunosuppressive therapies have been a hot topic of interest in the management of LADA. Phase II clinical studies of alum-formulated human recombinant glutamic acid decarboxylase 65-kDa isoform (GAD65) have shown that this molecule improves fasting C-peptide in the LADA population [17] and reduces the risk of insulin use; 1-α-hydroxyvitamin D3 (vitamin D3 [VD]) combined with insulin delays the course of LADA [18]. A series of clinical trials on LADA treatment options are in urgent need of statistical studies for analysis and evaluation.
Although LADA has received increasing attention as an autoimmune condition affecting a wide population, the academic understanding of LADA is still in the developmental stage, so the number of relevant high-quality randomized controlled trials (RCTs) is relatively small compared with typical RCTs on TIDM and T2DM. The existing analyses are mainly traditional meta-analyses [19] that focused on the clinical characteristics and diagnosis of LADA. Meta-analysis studies on residual β-cell function, an indicator used to assess the effectiveness of LADA treatment regimens, have not been reported in the literature. Therefore, we chose network meta-analysis to conduct a statistical study on the effectiveness of clinical interventions for LADA, with the overall aim to contribute to the current gap in research related to LADA treatment protocols and provide ideas for the best treatment options for LADA.
Methods
Literature Search
From inception to March 2023, searches of the PubMed, Cochrane Library, Embase and Web of Science databases were conducted by computer-based retrieval integrated with manual retrieval of related references. The systematic search we designed was a combination of keywords, and the free words strategy was formed by the following search terms: latent autoimmune diabetes in adults, slowly progressive type 1 insulin-dependent diabetes mellitus (SPIDDM), randomized controlled trial, clinical trial, treatment, among others. Search strategies carried out in the databases are listed in Table 1.
Inclusion and Exclusion Criteria
The inclusion criteria of the experimental studies were: (1) RCTs in any stage; (2) age of participant ≥ 18 years; (3) positivity of participant for glutamic acid decarboxylase antibody (GADAb), protein tyrosine phosphatase antibody (IA-2A) or islet cell antibody (ICA); (4) participants having no history of ketosis within the first 6 months after diagnosis; and (5) study efficacy outcomes available for glycosylated hemoglobin, type A1c (HbA1c), fasting blood glucose (FBG), fasting C-peptide (FCP), postprandial C-peptide (PCP), change from baseline in HbA1c (ΔHbA1c), and body mass index (BMI).
The criteria for excluded studies were: (1) non-RCTs, case reports, studies with insufficient data, conference reports, duplicated publications, summaries or systematic reviews; (2) women who were pregnant, lactating or planning on becoming pregnant; (3) participants with liver or kidney disorders or impaired hepatic/renal function; (4) participants with any severe systemic disease, such as cancer, stroke or heart failure, or having undergone recent surgery; (5) participants suffering from serious cardiovascular diseases or cerebrovascular conditions or having a history of ketoacidosis or having unstable or rapidly progressive diabetes nephropathy or neuropathy.
Data Extraction and Quality Assessment
Data extracted from the included studies were:
-
1.
Overview of the study, including clinical trial title, sponsors, clinical trial registration number, time of publication, journal and research location;
-
2.
Patient information and intervention, including sample size, sex, age, duration of disease, administration routes, specific medication, mean, standard deviation, dosage and length of follow-up;
-
3.
Outcomes, including effect, estimate, mean, mean difference (MD), confidence interval (CI).
Two researchers (Wanqing Wang and Fei Huang) evaluated the methodological quality of the included RCTs using Cochrane Collaboration’s tool for assessing risk of bias. This tool covers six domains, including selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias. Each domain was judged as “yes,” “unclear” or “no” to rate a grade as low (with no domain deemed “unclear” or “no”), moderate (with 1 domain deemed “unclear” or “no”) or high (with > 1 one weak rating) [20]. Quality assessment and investigation of publication bias were conducted by Review Manager 5 (RevMan 5.4; Cochrane Collaboration, Oxford, UK).
Both data extraction and quality assessment were independently performed by the same two reviewers who evaluated the methodological quality of the included RCTs. In case of disagreement, an independent third researcher (Chunchao Han) resolved inconsistencies through discussion.
Statistical Analysis
First, the heterogeneity test we selected was the effect model based on the I-square (I2) test and P-value. The heterogeneity test was based on the Knapp-Hartung modification. If I2 < 50% and the P-value > 0.05, there was no statistical heterogeneity in each study, and we used a fixed-effects model for the meta-analysis. However, an I2 > 50% and P-value < 0.05 indicated heterogeneity among the studies [21]. Subgroup analysis and sensitivity analysis were applied to further explain the appearance of heterogeneity [22]. The subgroup analysis was performed on the following: baseline C-peptide, the course of study, medicine type and treatment with or without insulin.
For the subgroup analysis on baseline C-peptide, we followed the international expert consensus recommendation of measuring C-peptide levels to determine treatment options and divided the data included in the study into three classifications according to low, medium and high levels of baseline C-peptide, respectively; these three groups corresponded to C-peptide levels < 0.3 nmol/L, C-peptide levels ≥ 0.3 and ≤ 0.7 nmol/L and C-peptide levels > 0.7 nmol/L, respectively [10]. We also categorized the results of this index into low, medium, and high levels again by calculating the tertiles of the baseline C-peptide index included in the literature for the evaluation of the subgroup analysis [23]. The two different classifications of baseline C-peptide are indicated in the Results section. The sensitivity analysis tested the stability of our analysis results or explained the reason why the literature became the cause of the heterogeneity. A network funnel plot was used to visually scrutinize the criterion of symmetry [24].
Second, STATA version 16.0 software (StataCorp LLC, College Station, TX, USA) was used to conduct the meta-analysis and produce network graphs. For the meta-analysis, continuous variables were expressed as MD, and the 95% CI was calculated for each effect indicator; I2 indicated the degree of heterogeneity among multiple studies. In the network graph, every intervention was represented as a node, and the sample sizes were indicated by node sizes. The thickness of the lines between the nodes reflected the number of included studies. After that, we conducted the node-splitting method to estimate the extent of inconsistency [25]. The fixed effects model (FEM) was applied if P > 0.05, while the random-effects model (REM) was put in place when P < 0.05.
Third, the surface under the cumulative ranking (SUCRA) was estimated for each management strategy [26]. Forest plots can visually imply the effectiveness of therapies [27]. The control group of studies performed by three-arm trials was divided by two (the odd number was divided by 2 after adding 1) before comparison with the other groups through forest plots, subgroup analysis and sensitivity analysis. All analyses were conducted in Stata version 16.0 software (StataCorp LLC).
Compliance with Ethics Guidelines
Ethics committee approval was not required for this network meta-analysis as it entailed the analysis of existing data extracted from previously published literature. A network meta-analysis does not entail direct contact with human participants or collection of new data. Instead, it involves a systematic statistical analysis of data that have already been collected and reported in previously published literature. Therefore, ethics committee permission was deemed unnecessary for this study. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Results
Baseline Characteristics of Included Studies
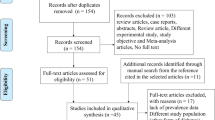
Electronic searches and manual searches undertaken in March 2023 initially identified 26,305 publications from the PubMed (n = 4587), Cochrane Library (n = 3794), Embase (n = 5251) and Web of Science (n = 12,673) databases. Of these 26,305 publications, 5846 were letters or reviews, 3191 were studies involving non-human animals, 13,217 were duplicates, 1336 were case reports and 2018 were marked as ineligible by the automation tools; these records were removed before screening. Subsequent assessment for eligibility eliminated were 22 non-RCT studies, 39 records unrelated to LADA, 214 records unrelated to the treatment of LADA, five records on different durations of the same study and nine records without data that could be compared with others. Ultimately, 14 articles covering approximately 23 eligible RCTs published from 1996 to 2021 were selected for inclusion in the network meta-analysis [12, 16,17,18, 28,29,30,31,32,33,34,35,36,37]. The flowchart of selection of studies is shown in Fig. 1.
One of articles included in our network meta-analysis is the study of Pozzilli et al. [12]. This was a subgroup analysis of three large multicenter randomized trials, but the subgroup data on LADA were not investigated in those three trials, so the results of this study were included in our analysis.
The study by Johansen et al. [34] was a prespecified exploratory analysis of a large trial, but because it was a double-blind randomized controlled study, it was also included in this meta-analysis.
The 14 articles covered a total of 15 management strategies: subcutaneous injections of GAD-alum; sitagliptin; pioglitazone; saxagliptin; insulin in combination with glibenclamide (insulin + glibenclamide); insulin; sulfonylureas; linagliptin; glimepiride; glibenclamide; insulin + vitamin D3; dulaglutide; insulin + sitagliptin; rosiglitazone; and insulin + sitagliptin (Fig. 2a, b). Five RCTs involved multiple ethnic groups (mainly Caucasians), two RCTs were conducted only in Caucasians and seven RCTs were conducted only in Asians. The papers selected for this network meta-analysis included 11 studies that were performed as two-arm trials, one as a three-arm trial, one as a four-arm trials and one as a five-arm trial. The basic characteristics and outcomes of each study are summarized in Table 2.
Network evidence for HbA1c and FCP of patients with LADA treated by different regimens. A HbA1c, B FCP. The size of each node is proportional to the number of participants assigned to the treatment. The thickness of the line is proportional to the number of trials between the corresponding drugs. HbA1c Glycosylated hemoglobin, FCP fasting C-peptide type A1c, LADA latent autoimmune diabetes in adults, VD 1-α-hydroxyvitamin D3 [1-α(OH)D3]
Quality Assessment
The Cochrane risk of bias assessment is presented in Fig. 3a, b. Eight trials were estimated to have a moderate risk of selection bias (allocation concealment), performance bias and detection bias. Two trials were regarded as having a moderate risk of both selection bias (allocation concealment) and detection bias. Two studies presented moderate risk in the detection bias only. In terms of the risks of selection bias (random sequence generation), attrition bias, reporting bias and other bias, all articles were appraised as low risk.
SUCRA Curves of Efficacy of 15 LADA Treatments
The cluster ranking analysis of SUCRA values showed that rosiglitazone and sitagliptin ranked first and second, respectively, in reducing HbA1c (Fig. 4a). The thiazolidinediones included in the study, whether as monotherapy or in combination therapy, ranked the highest in reducing HbA1c, such as rosiglitazone, insulin in combination with rosiglitazone and pioglitazone (Fig. 4a). Sulfonylureas ranked at the bottom (Fig. 4a). In terms of maintaining FCP levels, rosiglitazone ranked at the top, and the combination insulin + sulfonylureas or insulin alone ranked the worst (Fig. 4b). According to the ΔHbA1c, insulin achieved the greatest reduction of HbA1c, followed by dulaglutide, while the treatment regimen that included subcutaneous injections of GAD-alum was the worst in terms of lack of a significant reduction of HbA1c, with the exception of the blank control group with placebo (Fig. 4c). For maintaining PCP levels, rosiglitazone was the most effective, and insulin in combination with vitamin D3 was the least effective (Fig. 4d). Insulin in combination with sitagliptin and sitagliptin monotherapy ranked in the top two for reducing FBG, while the worst ranking in this regard was insulin in combination with sulfonylureas, and the second to last was sulfonylureas alone (Fig. 4e). The most effective regimen for BMI control was insulin and sitagliptin, and the least effective option was rosiglitazone or sulfonylureas (Fig. 4f).
SUCRA curves of HbA1c, FCP, ΔHbA1c, PCP, FBG and BMI of patients with LADA treated by 15 different regimens. A HbA1c, B FCP, C ΔHbA1c, D PCP, E FBG, F BMI. SUCRA surface under the cumulative ranking, HbA1c glycosylated hemoglobin, type A1c, FCP fasting C-peptide, ΔHbA1c change from baseline in HbA1c, PCP postprandial C-peptide, FBG fasting blood glucose, BMI body mass index, LADA latent autoimmune diabetes in adults, VD 1-α-hydroxyvitamin D3 (1-α(OH)D3), DPP-4 dipeptidyl peptidase-4
Heterogeneity, Inconsistency and Their Causes Among the 15 Treatments of LADA Included in the Studies
Glycosylated hemoglobin, type A1c
Seven studies reported the effects of different treatment regimens on HbA1c. Funnel plots suggested no significant publication bias, but heterogeneity was high (Fig. 5a), possibly related to the small number of included studies. Datasets for ≥ 8 treatment regimens in the form of funnel plots for HbA1c, FCP, PCP and BMI are shown in Fig. 5a–d, respectively. Egger’s test was used to assess publication bias with respect to HbA1c, and the results showed no publication bias. The inconsistency model test did not find inconsistency. The results of the node-splitting method to assess local inconsistency showed a great inconsistency between “insulin” and “insulin & rosiglitazone,” and the inconsistency also existed in the pair of nodes “sulfonylurea” and “rosiglitazone.” Because both two-arm and multiarm studies were present in the HbA1c group, the inconsistency test results were affected accordingly. Therefore, we paid more attention to the heterogeneity test results in the analysis of the HbA1c group data. The pooled analysis resulted in no significant difference between the application of different treatment regimens in terms of HbA1c, but there was significant heterogeneity (I2 = 82.9%) (Fig. 6a). The forest plot showed that only the effect values of Davis et al. [32] and Kobayashi et al. [35] fall to the right of the null line; in other words, the control group in these studies was more efficacious than the experimental group (Fig. 6a); in both studies, the experimental groups were administered sulfonylureas.
Funnel plots for HbA1c, FCP, PCP and BMI of patients with LADA treated by different regimens. A HbA1c, B FCP, C PCP, D BMI. HbA1c Glycosylated hemoglobin, type A1c, FCP fasting C-peptide, PCP postprandial C-peptide, BMI body mass index, LADA latent autoimmune diabetes in adults, VD 1-α-hydroxyvitamin D3 (1-α(OH)D3), se(WMD) standard error of the weighted mean difference
Forest plots of HbA1c, FCP, ΔHbA1c, PCP, FBG and BMI of patients with LADA treated by different regimens. A HbA1c, B FCP, C ΔHbA1c, D PCP, E FBG, F BMI. The treatment regimens for the experimental groups in the forest plot of HbA1c (A) for each study were, from top to bottom, sulfonylureas, sitagliptin, glibenclamide, insulin + sitagliptin, sulfonylureas, rosiglitazone, insulin + rosiglitazone, insulin + sitagliptin and insulin + rosiglitazone. The treatment regimens for the experimental groups in the forest plot of FCP (B) for each study were, from top to bottom, insulin + glibenclamide, sitagliptin, insulin + 1-α-hydroxyvitamin D3, insulin + sitagliptin, sulfonylureas, rosiglitazone, insulin + rosiglitazone, insulin + sitagliptin and insulin + rosiglitazone. The treatment regimens for the experimental groups in the forest plot of ΔHbA1c (C) for each study were, from top to bottom, glimepiride, placebo, dulaglutide + insulin glargine. The treatment regimens for the control groups in the forest plot of ΔHbA1c (C) for each study were, from top to bottom, linagliptin, saxagliptin, sitagliptin and sitagliptin. The treatment regimens for the experimental groups in the forest plot of PCP (D) for each study were, from top to bottom, insulin + 1-α-hydroxyvitamin D3, insulin + sitagliptin, sulfonylureas, rosiglitazone, insulin + rosiglitazone, insulin + sitagliptin and insulin + rosiglitazone. The treatment regimens for the experimental groups in the forest plot of FBG (E) for each study were, from top to bottom, insulin + glibenclamide, sitagliptin, glibenclamide + insulin and sitagliptin. The treatment regimens for the experimental groups in the forest plot of BMI (F) for each study were, from top to bottom, insulin + glibenclamide, glibenclamide, insulin + sitagliptin, sulfonylureas, rosiglitazone, insulin + rosiglitazone and insulin + sitagliptin. The treatment regimen for the control group in the forest plots of HbA1c (A), FCP (B), PCP (D), FBG (E) and BMI (F) for each literature was insulin. HbA1c Glycosylated hemoglobin, type A1c, FCP fasting C-peptide, ΔHbA1c change from baseline in HbA1c, PCP postprandial C-peptide, FBG fasting blood glucose, BMI body mass index, LADA latent autoimmune diabetes in adults, CI confidence interval, VD 1-α-hydroxyvitamin D3 (1-α(OH)D3), DPP-4 dipeptidyl peptidase-4.
Subgroup analysis suggested that participants with an intermediate FCP baseline grade exhibited better results with other treatment options than with insulin alone, with lower heterogeneity (I2 = 44.8%) (Fig. 7A,a; see also Electronic Supplementary Material [ESM] file). Other treatment options in the intermediate FCP baseline grade group included sitagliptin, rosiglitazone, insulin + rosiglitazone and sulfonylureas. Subgroup analysis of the treatment group showed that the treatment regimens involving thiazolidinediones were more effective and statistically significant than those with insulin alone, with less heterogeneity (I2 = 0.0%) (Fig. 7B,a; see also ESM file). However, since this result was obtained in three datasets from two trials [16, 37], the small sample size still has to be taken into account.
Subgroup analysis of HbA1c, FCP, PCP, FBG and BMI of patients with LADA treated by different regimens. A Subgroup analysis conducted at baseline FCP levels (stratified according to the international expert consensus), B subgroup analysis by class of drug regimens, C subgroup analyses conducted by whether insulin was chosen, D subgroup analyses conducted by duration of medication, E subgroup analysis of FCP conducted at baseline FCP levels (stratified according to the tertiles of baseline C-peptide index of the included literature). Aa HbA1c, Ab FCP, Ac PCP, A FBGd, Ae BMI; Ba HbA1c, Bb FCP, Bc PCP, Bd FBG, Be BMI; Ca HbA1c, Cb FCP, Cc PCP, Cd FBG, Ce BMI; Da FCP, Db PCP, Dc FBG, Dd BMI. The treatment regimens for the experimental groups are detailed in the Electronic Supplementary Material. The treatment regimen for the control group was insulin. HbA1c Glycosylated hemoglobin, type A1c, FCP fasting C-peptide, PCP postprandial C-peptide, FBG fasting blood glucose, BMI body mass index, LADA latent autoimmune diabetes in adults, VD 1-α-hydroxyvitamin D3 (1-α(OH)D3), DPP-4 Dipeptidyl peptidase-4, CI confidence interval
Sensitivity analysis of the publications included in the HbA1c group showed more stable overall results (Fig. 8a).
Sensitivity analysis of HbA1c, FCP, ΔHbA1c, PCP, FBG and BMI of patients with LADA treated by different regimens. A HbA1c, B FCP, C ΔHbA1c, D PCP, E FBG, F BMI. HbA1c Glycosylated hemoglobin, type A1c, FCP fasting C-peptide, ΔHbA1c change from baseline in HbA1c, PCP postprandial C-peptide, FBG fasting blood glucose, BMI body mass index, LADA latent autoimmune diabetes in adults, CI confidence interval
Fasting C-Peptide
A total of seven studies reported on the effects of different treatments in maintaining FCP levels. Funnel plots indicated no significant heterogeneity, publication bias or other bias (Fig. 5b). The results of Egger’s test also confirmed the absence of publication bias.
The results of the inconsistency model test and the estimation by the node-splitting method both showed no inconsistency. The results of the pooled analysis were that, overall, the treatment regimens in the experimental group were more effective than insulin alone in maintaining FCP, with less heterogeneity (I2 = 36.6%) (Fig. 6b). The treatment regimens in the experimental group included insulin + glibenclamide, sitagliptin, insulin + 1-α-hydroxyvitamin D3, insulin + sitagliptin, sulfonylurea, rosiglitazone and insulin + rosiglitazone. Subgroup analysis using the FCP baseline grade as a delineation criterion showed that the experimental group's dosing regimen was more effective and less heterogeneous (I2 = 0.0%) than insulin alone (Fig. 7A,b; see also ESM file). The subgroup analysis also reflected a homogeneous effect of β-cell function on the efficacy of certain drugs, an effect that was more pronounced with sulfonylureas: that is, participants with an intermediate grade of FCP at baseline were treated with insulin combined with sulfonylureas [31] (Fig. 7A,b; see also ESM file). The combination of the treatments had effect values falling to the left of the null line (Fig. 7A,b; see also ESM file), suggesting a lower benefit than with insulin alone, while the opposite was shown in participants with high baseline levels of FCP [16]. If the results of this index are stratified into three levels (low, medium and high) according to the tertiles of baseline C-peptide index reported in the included studies, the trend of better efficacy in participants with better β-cell function would also be traced for DPP-4 inhibitors [28, 33] (Fig. 7E; see also ESM file). However, it is difficult to observe a propensity effect on the efficacy of thiazolidinediones, although given the limited sample size, this conclusion needs to be confirmed by additional high-quality clinical studies (Fig. 7E; see also ESM file). The results of the subgroup analysis, based on the classification of the different classes of drugs showed that the treatment regimens involving thiazolidinediones could be more effective than that with insulin alone, but with greater heterogeneity (I2 = 52.3%), which might be related to the small number of included studies (Fig. 7B,b; see also ESM file). This subgroup analysis did not support a conclusion that there was a statistically significant difference between regimens involving sulfonylureas or DPP-4 inhibitors and insulin alone in terms of maintaining FCP levels. The results of this analysis were probably related to the fact that participants who received different treatment regimens also had differences in their FCP at baseline. The results of the subgroup analysis conducted according to whether insulin was applied showed that the regimens in which other drugs were combined with insulin were more effective than insulin alone, and this result was less heterogeneous (I2 = 0.0%) (Fig. 7C,b;; see also ESM file). In contrast, there was no significant difference between using other drugs alone and using insulin alone in terms of maintaining the FCP index (Fig. 7C,b; see also ESM file). The results of the subgroup analysis carried out on the duration of medication showed that the experimental group had probably a more favorable regimen than using insulin alone for maintaining FCP levels when the medication was administered for > 1 year, with a small heterogeneity value (I2 = 44.5%) (Fig. 7D,a; see also ESM file). Sensitivity analysis evaluation of the FCP group showed that the combined effect size did not change significantly with individual study exclusion, indicating that the data in this group were relatively robust (Fig. 8b). However, excluding any of the following datasets, which included two sets of data from Yang et al. [16], Li et al. [18], Yang et al. [36] or Zhou et al. [37], respectively, the combined results of the remaining studies were not statistically significant and were not consistent with the original combined results (MD = 0.072, 95% CI = 0.002–0.143), reflecting the large dispersion of the data in this group, which impacts the stability of the results (Fig. 8b).
△HbA1c
Three studies reported the effects of different treatment regimens on ΔHbA1c, and funnel plots demonstrated no significant heterogeneity, publication bias or other bias. The Egger test results indicated no publication bias. The inconsistency model test did not find any inconsistency. Pooled analysis showed large heterogeneity (I2 = 87.5%), and there was no significant difference between the experimental group's dosing regimen and that of the control group in terms of the ΔHbA1c (Fig. 6c). The medication regimens in the experimental group were glimepiride, dulaglutide, glargine and placebo as a blank control, and those of the control groups were DPP-4 inhibitors. Sensitivity tests showed a greater effect on the pooled effect size in the study of Buzzetti et al. [30], which is consistent with the study design of these authors, which compared DPP-4 inhibitors with placebo as a control study (Fig. 8c).
Postprandial C-Peptide
A total of five papers reported the effects of different treatment regimens on the maintenance of PCP. The overall sample size was small for the funnel plot, but there was no significant heterogeneity, publication bias or other bias (Fig. 5c). The Egger test indicated the presence of publication bias or small sample bias. We used the trim-and-fill method to estimate the robustness of the combined results. The pooled results for heterogeneity (P = 0.000) and effect indicators before filling in data from the literature (MD = 0.764, 95% CI = 0.154–1.374) were not reversed compared to the pooled results for heterogeneity (P = 0.000) and effect indicators after filling the data from the four virtual studies (MD = 0.927, 95% CI = 0.528–1.631). The pooled results were stable with reversal. Therefore, the effect of publication bias was not significant, and the results were quite robust. The assessment of both the inconsistency model and the node-splitting method suggested that there was no significant inconsistency. The results of the pooled analysis showed that the treatment regimens in the experimental group had a more pronounced effect on the maintenance of PCP than using insulin alone in the control group, but with greater heterogeneity (I2 = 93.4%) (Fig. 6d). The treatment regimens in the experimental group included insulin + 1-α-hydroxyvitamin D3, insulin + sitagliptin, sulfonylureas, rosiglitazone, insulin + rosiglitazone, insulin + sitagliptin and insulin + rosiglitazone. The subgroup analysis using FCP baseline grade as a delineation criterion showed that participants with high FCP baseline grade were better off using other treatment regimens than insulin alone, but this result included data from only one study with high heterogeneity (I2 = 66.4%) (Fig. 7A,c; see ESM file). The group with moderate FCP baseline showed that the treatment regimens in the experimental and control groups were not statistically significant (Fig. 7A,c; see ESM file). The results of the treatment subgroup analysis showed higher PCP levels in the population treated with thiazolidinediones compared to that treated with insulin alone, but there was high heterogeneity in this group (I2 = 85.1%) due to the small sample size (Fig. 7B,c; see ESM file). It is noteworthy that although this subgroup showed that the choice of sulfonylureas appeared to be more effective than insulin in maintaining PCP, the article with a choice of sulfonylureas had the highest FCP baseline level among the five papers that reported the effect of PCP maintenance. Therefore, we prefer to hypothesize that this result indicates the importance of preserving β-cell function for LADA treatment. The results of the subgroup divided by duration of medication showed that participants on medication for > 1 year had better maintenance of the PCP index with other treatment regimens than insulin alone, but with greater heterogeneity (I2 = 89.0%) (Fig. 7D,b; see ESM file). Subgroup analysis using the presence or absence of insulin as a delineator did not yield statistically significant results (Fig. 7C,c; see ESM file). The sensitivity analysis results were robust (Fig. 8d).
Fasting Blood Glucose
Four studies reported on the effects of different drug regimens in reducing FBG. Funnel plots showed no significant heterogeneity, publication bias or other bias. Egger’s test revealed no publication bias. The results of the inconsistency model test showed no inconsistency. The pooled analysis resulted in no difference in the treatment for the experimental group compared to using insulin alone for the control group in terms of control of FBG, with high heterogeneity (I2 = 85.6%) (Fig. 6e). The regimens in the experimental group were insulin + glibenclamide, sitagliptin, glibenclamide and insulin + sitagliptin. Subgroup analysis conducted at baseline FCP levels showed no difference in treatment regimens between the experimental and control groups for participants with moderate baseline FCP levels (Fig. 7A,d;; see ESM file). The regimens of the experimental group of the subgroup analysis were insulin + glibenclamide, sitagliptin and insulin + sitagliptin. Subgroup analysis by class of drug regimens showed that the regimen with sulfonylureas was less effective than insulin in reducing FBG, while the regimen with DPP-4 inhibitors yielded a better reduction of FBG than insulin with less heterogeneity (I2 = 0.0%) (Fig. 7B,d; see ESM file). Due to the paucity of literature reporting the control of FBG, no valuable subgroup analysis could be developed regarding the duration of dosing (Fig. 7D,c; see ESM file) and whether the drug regimens included insulin (Fig. 7C,d; see ESM file). The sensitivity analysis showed a steady result (Fig. 8e).
Body Mass Index
Five papers reported the effects of different treatment regimens on reduction of the BMI. No significant heterogeneity, publication bias or other bias was found in the funnel plot (Fig. 5d). The results of Egger’s test indicated no publication bias. The outcomes of the inconsistency model test and the assessment of the node-splitting method showed that there was no inconsistency. The pooled analysis showed no statistically significant differences in BMI reduction between the experimental and control groups, and the heterogeneity of this outcome was low (I2 = 0.0%) (Fig. 6f). The treatment regimens in the experimental group included insulin + glibenclamide, glibenclamide, insulin + sitagliptin, sulfonylurea, rosiglitazone and insulin + rosiglitazone. The results of the subgroup analysis using the FCP baseline class as the criterion for dividing the groups showed no difference in the control of BMI between the experimental and control treatment regimens, regardless of whether the FCP baseline grade was moderate or high (Fig. 7A; see ESM file). Interestingly, in this subgroup, the single effect value in the literature, in the study of Yang et al. [16], all fell to the right of the null line, although the use of insulin was more effective than that of sulfonylureas, rosiglitazone or insulin + rosiglitazone, and the baseline FCP grade of the control group with insulin alone was moderate, which was lower than the baseline FCP grades of both the sulfonylurea and rosiglitazone groups; this result confirms the effect of insulin in controlling BMI (Fig. 7A; see ESM file). In addition, although the effect values reported by Cabrera-Rode et al. [31] showed that insulin + glibenclamide was more effective in reducing BMI than insulin alone, the baseline FCP grade in their control group was low. Kobayashi et al. [35] showed that even though β-cell function was good in both the experimental and control groups, insulin was still more effective than glibenclamide (Fig. 7A; see ESM file), and the experimental group medication regimens also involved glibenclamide. The confidence intervals of both studies involving glibenclamide were relatively large (Fig. 7A,e; see ESM file), suggesting that there may be large fluctuations in the efficacy of sulfonylurea-involved regimens, which need to be clarified by more studies.
Subgroup analysis by type of treatment method showed that regimens containing DPP-4 inhibitors were probably more effective than insulin alone in controlling BMI, with less heterogeneity (I2 = 0.0%), but the literature is sparse for this dataset, and it is now generally accepted that DPP-4 inhibitors are generally weight-neutral [11, 38, 39]. Therefore, more literature data are needed to support this result (Fig. 7B,e; see ESM file). Subgroup analyses conducted by duration of medication (Fig. 7D,d; see ESM file) or by whether insulin was chosen (Fig. 7C,e; see ESM file) showed that duration of medication or whether insulin was included in the treatment regimen did not have a statistically significant effect on the effect on treatment and control groups in reducing BMI. Sensitivity analyses showed that only the exclusion of data from Yang et al. [36] changed the pooled effect size from positive to negative, while the effect size of the remaining data from the literature and the 95% CI did not change significantly due to the exclusion of individual studies, making the overall results fairly stable (Fig. 8f).
Discussion
In this study we evaluated the effects of insulin, sulfonylurea, DPP-4 inhibitors, thiazolidinediones, GLP-1RAs, insulin in combination with 1-alpha-hydroxyvitamin D3 (insulin + vitamin D], subcutaneous injections of GAD-alum and combination regimens of the abovementioned classes of drugs on HbA1c, FCP, ΔHbA1c, PCP, FBG and BMI in the LADA population. The SUCRA plots and pooled analysis of all studies showed that the treatment regimens involving thiazolidinediones were the most effective in reducing HbA1c (Figs. 4a, 6a) and maintaining FCP (Figs. 4b, 6b) and PCP (Figs. 4d, 6d) levels, but the least effective in reducing BMI (Figs. 4f, 6f). Insulin + sitagliptin was the most effective treatment regimen for reducing FBG (Figs. 4e, 6e) and BMI (Figs. 4f, 6f). The results of the subgroup analysis of treatment were similar to those of the pooled analysis. The subgroup analysis of FCP baseline grade showed that participants with better β-cell function could be better off with a treatment regimen other than insulin alone with respect to these indicators of HbA1c (Fig. 7A,a; see ESM file), PCP (Fig. 7Ac; see ESM file) and FCP (Fig. 7A,b; see ESM file). This result is in line with the guidelines introduced for the selection of treatment regimens according to C-peptide levels [10]: that is, with high C-peptide level, treatment with drugs other than insulin or with other drugs combined with insulin is preferable, while in those with poor C-peptide levels, insulin is more suitable. Subgroup analysis of medication duration showed that in terms of maintenance of FCP (Fig. 7D,a; see ESM file) and PCP (Fig. 7D,b; see ESM file), participants on medication for > 1 year would probably benefit from enhanced efficacy with a regimen other than insulin alone. The results of the subgroup analysis of whether the treatment regimens included insulin showed that the treatment regimens of insulin combined with other drugs would be more effective in maintaining FCP than insulin alone (Fig. 7C,b; see ESM file). Sensitivity analysis showed that most of the study data were robust, with two exceptions (Fig. 8c, f). in the study by Buzzetti et al. [30], the ΔHbA1c index (Fig. 8c) affected the robustness of the group by using placebo rather than other therapeutic agents as a control group. The BMI group (Fig. 8f) of Yang et al. [36] had a larger number of participants, and the mean BMI of the control group was higher than that of the experimental group, a feature that had an impact on the robustness of the results, in contrast to most of the data characteristics of this group. Our study was not subject to publication bias, and there were no inconsistencies that would significantly affect the results of the data analysis.
Insulin is still being used as the first choice of pharmacological intervention in the treatment of adult-onset autoimmune (AOA) diabetes [2]. Exogenous insulin effectively preserves β-cell function by resting pancreatic β-cells and thus protects these cells from damage by immune and metabolic mechanisms [40, 41]. One of the most immediate concerns regarding the use of insulin in the LADA population is the timing of initiation of insulin therapy [2, 42]. The latest international expert consensus on the treatment of LADA suggests measuring C-peptide concentrations to determine the treatment regimen: that is, a multiple-insulin regimen is recommended for participants with C-peptide levels < 0.3 nmol/L; for participants with C-peptide levels ≥ 0.3 nmol/L and ≤ 0.7 nmol/L, it is recommended that a combination regimen of other therapies with insulin be considered that does not lead to deterioration of β-cell function; in the case of C-peptide > 0.7 nmol/L, it is suggested that treatment be provided according to T2DM treatment [10]. Our subgroup analysis of FCP baseline levels divided the data included in the study into three groups: low, medium and high FCP baseline levels according to the above-mentioned C-peptide classification criteria. The results of this subgroup analysis showed that among the three indicators of HbA1c, PCP and FCP, the experimental group could be more effective in maintaining FCP and PCP levels and reducing HbA1c than the control group with insulin alone when the baseline level of FCP was in the medium and/or high class. This difference was also statistically significant and less heterogeneous for FCP indicator, and the number of papers using this indicator as an assessment indicator was large. The results of our subgroup analysis confirmed the statistical reliability and validity of the expert consensus statement's recommendation to adjust the medication regimen for patients with AOA diabetes according to C-peptide levels. In addition, despite the generally recognized effectiveness of insulin in regulating, among other things, β-cell failure in the LADA population [2, 19], the results of our cluster ranking analysis of SUCRA values indicated that insulin was moderately effective in improving all indicators. In contrast, insulin combined with other treatments ranked high in terms of SUCRA values. In particular, the combination of insulin + sitagliptin ranked top in the group in terms of efficacy in reducing FBG and BMI. More notably, the data from the literature included in the analysis of both FBG and BMI had medium to high FCP baseline grades, but the differences in FCP baseline grades were not statistically significant in the comparison of treatment regimens for FBG and BMI. Few RCTs have been able to confirm the efficacy of insulin combined with sitagliptin for the treatment of LADA [28, 36], so further clinical studies are needed to determine whether insulin + sitagliptin together can broadly help improve FBG and BMI levels in the LADA population with different β-cell functions. The pooled analysis likewise demonstrated that insulin may not be optimal in terms of the maintenance effects for FCP and PCP. Subgroup analysis of FCP with respect to whether insulin was applied showed that the combination regimens of insulin were more effective than insulin alone. Regarding the benefits of insulin ancillary drugs compared with increasing insulin dose in terms of efficacy, it has been suggested that insulin ancillary drugs can not only improve glycemic control but also better counteract insulin adverse events in the treatment of T1DM [8]. However, more prospective RCTs are needed to provide more scientific and reasonable evidence on the benefits of the combination regimens of insulin in the treatment of LADA. Overall, the results of our meta-analysis suggested that the combination of insulin and other treatment regimens was more likely to be more effective in terms of improvement in several indicators, such as FCP and PCP, compared to treatment with insulin alone. This may be related to the generally high baseline levels of FCP reported in the published data we included in our study, so this speculation remains to be proven by data from RCTs with sufficient follow-up time.
Sulfonylureas are not recommended for the treatment of LADA [10, 19, 43]. This class of drugs stimulate insulin secretion by inhibiting KATP channels, thereby causing depolarization of the β-cell membrane and opening Ca2+ channels, and thus causing Ca2+ inward flow. However, this process triggers a reduction in the number of functional KATP channels in the plasma membrane, a decrease in β-cells and accelerated death which, taken together, lead to a progressive impairment of the acute insulinotropic effects of sulfonylureas [44]. Several RCTs have shown that compared to insulin and DPP-4 inhibitors, sulfonylureas provide poorer metabolic control and reduce C-peptide levels more rapidly [16, 34, 41]. These findings are consistent with the results of our meta-analysis. Cluster ranking analysis of SUCRA values showed that the treatment regimens involving sulfonylureas were less effective in improving several indicators, such as HbA1c, ΔHbA1c, FBG and BMI, compared to other treatment regimens. In particular, in terms of the control of FBG, subgroup analysis of treatment showed that the experimental group with treatment regimens containing sulfonylureas were less effective in reducing FBG than the control group with insulin alone, and the difference was statistically significant. The data from the literature in the forest plot of HbA1c also suggested that only the experimental group with sulfonylureas had effect values falling to the right of the null line; that is, the control group treated with insulin alone was more efficacious than the experimental group [32, 35] (Fig. 6a). This difference between sulfonylureas and other treatment regimens inevitably rendered the results of this pooled analysis highly heterogeneous (I2 = 82.9%), which also indicated its nondominance in the modulation of HbA1c (Fig. 6a). Although the SUCRA plots for FCP (Fig. 4b) and PCP (Fig. 4d) showed that sulfonylureas alone ranked second, volunteers on sulfonylureas alone had the highest baseline FCP in the data for both indices and a clear advantage over the baseline levels of FCP in the other groups. Moreover, the sulfonylurea combination regimens were the least effective in terms of FCP maintenance. Thus, sulfonylureas did not appear to be as effective in terms of FCP and PCP maintenance based on the SUCRA graph. The results of our subgroup analysis in which FCP baseline grade was the delineation criterion further confirmed the conjecture that subjects with higher FCP baseline were more suitable for a combined insulin regimen, while subjects with low FCP baseline were more suitable for treatment with insulin alone. This trend was most pronounced in the experimental group in which sulfonylureas and DPP-4 inhibitors were involved. We are therefore more inclined to hypothesize that the results of our analysis suggest the possibility that participants with better residual β-cell function experience better treatment efficacy with a combined insulin regimen, but they do not suggest that sulfonylurea treatment is appropriate for the LADA population. These findings corroborate the current recommendations for the selection of LADA treatment regimens [10].
Thiazolidinediones are a class of insulin sensitizers that act as agonists of highly selective peroxisome proliferator-activated receptor-γ (PPARγ), thus increasing the response of target tissues of insulin action, such as skeletal muscle and liver, to insulin, and exert a significant ameliorative effect on insulin resistance while lowering glucose levels [45]. The cluster ranking analysis of SUCRA values conducted in this study showed that the treatment regimens involving thiazolidinediones ranked first in the control of HbA1c (Fig. 4a), FCP (Fig. 4b) and PCP (Fig. 4d) but ranked last in the control of BMI (Fig. 4f) [16, 37]. Similarly, in a subgroup analysis of treatment by drug class, a statistically significant advantage of thiazolidinediones over insulin alone in the regulation of HbA1c (Fig. 7B,a; see ESM file) and PCP (Fig. 7B,c; see ESM file) was shown [16, 37]. However, all of these observations were limited by the small number of available clinical studies and small sample sizes. Because of the paucity of relevant literature, it was not possible to clarify whether there was a correlation between the efficacy of thiazolidinediones and basal β-cell function in the LADA population. Thiazolidinediones have a promising future in the treatment of LADA, but refining them into a mature therapeutic strategy for the effective management of LADA still requires more high-quality clinical trials.
GLP-1RAs are relatively emergent hypoglycemic agents that lower blood glucose by activating the GLP-1 receptor. GLP-1 is an enteroglucagon that promotes insulin secretion from pancreatic β-cells in a glucose concentration-dependent manner and inhibits glucagon secretion; it is also able to play a role in delaying gastric emptying and in weight loss [13, 14, 46, 47]. Few clinical RCTs have been performed on the use of GLP-1RA in the treatment of LADA, and our meta-analysis includes study data on GLP-1RA and the index ΔHbA1c. Cluster ranking analysis of SUCRA values showed that the effectiveness of GLP-1RAs in modulating ΔHbA1c was relatively very effective, second only to insulin [12] (Fig. 4c). Based on the pooled analysis of ΔHbA1c, it was also possible to determine that GLP-1RAs contributed more to the regulation of ΔHbA1c levels than did DPP-4 inhibitors (Fig. 6c), but since the literature included in this study excluded some participants receiving insulin therapy [12, 48,49,50,51], this result actually tended more to indicate that GLP-1RA would benefit LADA patients who had a certain amount of islet function. A recent study found a protective effect of a regimen of liraglutide + anti-interleukin (IL)-21 on β-cells in T1DM [52], but liraglutide alone did not demonstrate such an effect. The results of our analysis similarly did not support the notion that GLP-1RAs had an effect on β-cell function. Due to the lack of prospective, large-scale, long-term RCTs on GLP-1RAs with respect to the ability of this drug class to control metabolic abnormalities and maintain β-cell function in the LADA population, there is a lack of supportive data on the effect of GLP-1RAs in the treatment of LADA.
Immunological intervention studies for the LADA population were dominated by a study assessing the safety and efficacy of GAD-alum using subcutaneous injections of GAD-alum compared to placebo [17]. The most recent phase 2 findings of this study were that a 20-μg dose of GAD-alum increased FCP in this group of participants over 5 years compared with placebo, and did not impair β-cell function [17]. In the present study, a cluster ranking analysis of SUCRA values regulating ΔHbA1c showed that although subcutaneous injections of GAD-alum were more effective than placebo, they still fell short compared to other treatment options and even ranked behind sulfonylureas [17] (Fig. 4c). The findings with respect to this immune intervention therapy are still limited by small sample trials, and the results need to be validated by more high-quality RCTs with larger sample sizes. The results of systematic studies on, for example, effective injection dose and periodicity, are still unclear, so there is still far-reaching space for exploration.
VD is thought to improve immune imbalance. It has been shown that the VD receptor (VDR) is a key regulator of β-cell survival [53]. The results of a multicenter RCT showed that the addition of vitamin D3 to the medication regimen of saxagliptin and conventional therapy would be more beneficial in protecting the function of β-cells in the LADA population [54]. We could perceive on the cluster ranking plot of the SUCRA values of this study that the regimens involving vitamin D3 ranked high in the hierarchical ranking of maintaining FCP levels (Fig. 4b), even before the introduction of DPP-4 inhibitors, although the effect on PCP maintenance was not as outstanding [18] (Fig. 4d). The conclusions drawn on the pooled analysis mirrored the results of the cluster ranking of SUCRA values (Fig. 6b, d). However, there were also some studies stating that VD was associated with insulin resistance in T2DM but not with LADA; even serum concentrations of VD were not significantly correlated with C-peptide in LADA or T2DM [55, 56]. Undoubtedly, the ability of VD to maintain β-cell function and improve immune and metabolic imbalances in the LADA population also needs to be confirmed by many more large RCTs.
The subgroup analysis of this study also demonstrated that the various regimens of LADA were influenced by the duration of the experiment, particularly for PCP (Fig. 7D,b; see ESM file). The analysis of its temporal subgroup showed that a study duration of > 1 year had a statistically significant effect on the results, and that the heterogeneity tended to decrease [16, 28, 36, 37]. This finding provides data-supported recommendations for the optimal time to conduct RCT studies exploring the therapeutic implications of LADA.
The results of this network meta-analysis are significant because:
-
(1)
It is the first time that a comprehensive and systematic network meta-analysis of treatment options for LADA was conducted, including a total of 15 drug regimens for the treatment of LADA, with combination drug treatments, drugs as monotherapy, injectable drugs and oral drugs. This analysis not only assessed the statistical quality of the relevant literature on the treatments of LADA, but also provided recommendations for designing research protocols and research directions for conducting future studies of the regimens of LADA.
-
(2)
Cluster ranking analysis of SUCRA values, forest plots, subgroup analysis, sensitivity analysis, funnel plots, publication bias and other methods, and the effects of various treatment regimens on multiple indicators reflecting different meanings of LADA were analyzed and evaluated from multiple perspectives, providing intuitive data support for the clinical choices of physicians and the LADA population.
-
(3)
The study was not affected by publication bias or other bias.
-
(4)
The analysis provided statistical evidence for the treatment regimens of other drugs combined with insulin. The strategy of insulin in combination with other drugs may be expected to alleviate the complications caused by long-term insulin application in the LADA population.
-
(5)
Subgroup analysis was conducted with FCP baseline, incorporating the C-peptide concentration delineation method proposed by expert consensus [10], and the effects of various treatment regimens on residual β-cell function were explored.
-
(6)
Subgroup analysis of medication duration demonstrated the association between β-cell function and medication duration.
However, this network meta-analysis also has the following limitations:
-
(1)
RCT studies of LADA are still in their infancy, and there are few high-quality RCTs related to LADA; small clinical studies predominate the literature. Therefore, the published data included in our study have the limitation of a small sample size in general. For example, this inevitably brought heterogeneity to this meta-analysis and had an impact on the stability of the relevant data under the BMI index. This can be demonstrated in the results of sensitivity analysis of BMI [16, 28, 31, 35, 36].
-
(2)
The different participant characteristics and durations of experimental studies and the complexity of drug administration in the studies included in the analysis make certain data show a tendency to be widely scattered, and the variability of the various protocols needs to be further clarified.
-
(3)
In order to elaborate as comprehensively as possible on the efficacy of various treatment regimens in the LADA population, the included studies spanned a wide range of sample sizes, follow-up durations and interventions, which adds to the intrinsic challenge for a uniform definition of LADA.
-
(4)
The design of RCT study protocols regarding interventions for LADA has progressed with the increasing understanding of LADA, with some of the older clinical studies on LADA having limitations in terms of study strategy, and even a few studies using sulfonylureas as interventions. Although the results of our analysis do not recommend the use of sulfonylureas in the LADA population, there is no doubt that RCTs with more acceptable design and methodology need to be conducted to better compare the effects of various treatment options in this population.
-
(5)
Due to the paucity of reports of prospective RCTs in the LADA population, our analysis cannot avoid certain limitations. Therefore, there is still a great deal of room for development of research on treatment regimens for LADA. In particular, the network meta-analysis of treatment regimens for LADA can only be made more scientifically sound by increasing the amount of data from specifically designed prospective RCTs in this population.
Conclusion
Overall, the results of this network meta-analysis suggest that insulin has a significant advantage in modulating ΔHbA1c in the LADA population. The combination insulin + DPP-4 inhibitors has the most significant effect in reducing FBG and BMI, and treatment regimens involving thiazolidinediones achieve the best effects in modulating HbA1c, FCP and PCP. GLP-1RAs, immune interventions and regimens that incorporate vitamin D still require additional prospective studies with large samples and long-term follow-ups to obtain clearer results. Our findings support the recommendation of an international expert consensus that divides C-peptide concentrations into three groups corresponding to different treatment regimens: C-peptide levels < 0.3 nmol/L, C-peptide levels ≥ 0.3 nmol/L, ≤ 0.7 nmol/L and C-peptide levels > 0.7 nmol/L [10]. In addition, a longer duration of therapy may be more highly beneficial to the LADA population in terms of maintaining β-cell function.
References
Petreski H. Misdiagnosed patient with latent autoimmune diabetes in adults (case report). Georgian Med News. 2018;277:31–5.
Buzzetti R, Maddaloni E, Gaglia J, Leslie RD, Wong FS, Boehm BO. Adult-onset autoimmune diabetes. Nat Rev Dis Primers. 2022;8(1):63. https://doi.org/10.1038/s41572-022-00390-6.
Leslie RD, Kolb H, Schloot NC, et al. Diabetes classification: grey zones, sound and smoke: action LADA 1. Diabetes Metab Res Rev. 2008;24(7):511–9. https://doi.org/10.1002/dmrr.877.
World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. 1999. https://apps.who.int/iris/handle/10665/66040. Acessed 17 June 2012.
Irvine WJ, McCallum CJ, Gray RS, Duncan LJ. Clinical and pathogenic significance of pancreatic-islet-cell antibodies in diabetics treated with oral hypoglycaemic agents. Lancet. 1977;1(8020):1025–7. https://doi.org/10.1016/s0140-6736(77)91258-2.
Turner R, Stratton I, Horton V, et al. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet. 1997;350(9087):1288–93. https://doi.org/10.1016/s0140-6736(97)03062-6. (Erratum. In: Lancet 1998;351(9099):376).
van Deutekom AW, Heine RJ, Simsek S. The islet autoantibody titres: their clinical relevance in latent autoimmune diabetes in adults (LADA) and the classification of diabetes mellitus. Diabet Med. 2008;25(2):117–25. https://doi.org/10.1111/j.1464-5491.2007.02316.x.
Dellepiane S, Ben Nasr M, Assi E, et al. Sodium glucose cotransporters inhibitors in type 1 diabetes. Pharmacol Res. 2018;133:1–8. https://doi.org/10.1016/j.phrs.2018.04.018.
Cernea S, Buzzetti R, Pozzilli P. Beta-cell protection and therapy for latent autoimmune diabetes in adults. Diabetes Care. 2009;32(Suppl 2):S246–52. https://doi.org/10.2337/dc09-S317.
Buzzetti R, Tuomi T, Mauricio D, et al. Management of latent autoimmune diabetes in adults: a consensus statement from an international expert panel. Diabetes. 2020;69(10):2037–47. https://doi.org/10.2337/dbi20-0017.
Morieri ML, Raz I, Consoli A, et al. Short-term effectiveness of dapagliflozin versus DPP-4 inhibitors in elderly patients with type 2 diabetes: a multicentre retrospective study. J Endocrinol Invest. 2023;46(7):1429–39. https://doi.org/10.1007/s40618-022-02002-2.
Pozzilli P, Leslie RD, Peters AL, et al. Dulaglutide treatment results in effective glycaemic control in latent autoimmune diabetes in adults (LADA): a post-hoc analysis of the AWARD-2, -4 and -5 trials. Diabetes Obes Metab. 2018;20(6):1490–8. https://doi.org/10.1111/dom.13237.
Berra CC, Rossi MC, Mirani M, et al. Real world effectiveness of subcutaneous semaglutide in type 2 diabetes: a retrospective, cohort study (Sema-MiDiab01). Front Endocrinol (Lausanne). 2023;18(13):1099451. https://doi.org/10.3389/fendo.2022.1099451.
Bendotti G, Montefusco L, Lunati ME, et al. The anti-inflammatory and immunological properties of GLP-1 receptor agonists. Pharmacol Res. 2022;182:106320. https://doi.org/10.1016/j.phrs.2022.106320.
Jones AG, McDonald TJ, Shields BM, et al. Markers of β-cell failure predict poor glycemic response to GLP-1 receptor agonist therapy in type 2 diabetes. Diabetes Care. 2016;39(2):250–7. https://doi.org/10.2337/dc15-0258.
Yang Z, Zhou Z, Li X, Huang G, Lin J. Rosiglitazone preserves islet beta-cell function of adult-onset latent autoimmune diabetes in 3 years follow-up study. Diabetes Res Clin Pract. 2009;83(1):54–60. https://doi.org/10.1016/j.diabres.2008.09.044.
Agardh CD, Lynch KF, Palmér M, Link K, Lernmark A. GAD65 vaccination: 5 years of follow-up in a randomised dose-escalating study in adult-onset autoimmune diabetes. Diabetologia. 2009;52(7):1363–8. https://doi.org/10.1007/s00125-009-1371-2.
Li X, Liao L, Yan X, et al. Protective effects of 1-alpha-hydroxyvitamin D3 on residual beta-cell function in patients with adult-onset latent autoimmune diabetes (LADA). Diabetes Metab Res Rev. 2009;25(5):411–6. https://doi.org/10.1002/dmrr.977.
Brophy S, Davies H, Mannan S, Brunt H, Williams R. Interventions for latent autoimmune diabetes (LADA) in adults. Cochrane Database Syst Rev. 2011;2011(9):CD00165. https://doi.org/10.1002/14651858.CD006165.pub3.
Higgins JPT, Thomas J, Chandler J, et al. (editors). Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester: John Wiley & Sons; 2019.
Nowrouzi-Sohrabi P, Tabrizi R, Jalali M, et al. Effects of diacerein intake on cardiometabolic profiles in type 2 diabetics: a systematic review and meta-analysis of clinical trials. Curr Med Chem. 2021;28(4):840–52. https://doi.org/10.2174/0929867327666200728134755.
Ko Y, Lee WJ, Park JH, et al. Diagnostic sensitivity and specificity of 2-mSv CT vs. conventional-dose CT in adolescents and young adults with suspected appendicitis: post hoc subgroup analysis of the LOCAT data. Eur Radiol. 2020;30(8):4573–85. https://doi.org/10.1007/s00330-020-06811-y.
Iwamoto N, Matsui A, Kazama H, Oura T. Subgroup analysis stratified by baseline pancreatic β-cell function in a japanese study of dulaglutide in patients with type 2 diabetes. Diabetes Ther. 2018;9(1):383–94. https://doi.org/10.1007/s13300-017-0346-4.
Altobelli E, Del Negro V, Angeletti PM, Latella G. Low-FODMAP diet improves irritable bowel syndrome symptoms: a meta-analysis. Nutrients. 2017;9(9):940. https://doi.org/10.3390/nu9090940.
Guo T, Ren L, Wang Q, Li K. A network meta-analysis of updated haemostatic strategies for hysterectomy. Int J Surg. 2016;35:187–95. https://doi.org/10.1016/j.ijsu.2016.10.002.
Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–71. https://doi.org/10.1016/j.jclinepi.2010.03.016.
Ardeshirlarijani E, Tabatabaei-Malazy O, Mohseni S, Qorbani M, Larijani B, Baradar JR. Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: a meta-analysis of randomized trials. Daru. 2019;27(2):827–37. https://doi.org/10.1007/s40199-019-00302-2.
Wang X, Yang L, Cheng Y, et al. Altered T-cell subsets and transcription factors in latent autoimmune diabetes in adults taking sitagliptin, a dipeptidyl peptidase-4 inhibitor: a 1-year open-label randomized controlled trial. J Diabetes Investig. 2019;10(2):375–82. https://doi.org/10.1111/jdi.12873.
Awata T, Shimada A, Maruyama T, et al. Possible long-term efficacy of sitagliptin, a dipeptidyl peptidase-4 inhibitor, for slowly progressive type 1 diabetes (SPIDDM) in the stage of non-insulin-dependency: an open-label randomized controlled pilot trial (SPAN-S). Diabetes Ther. 2017;8(5):1123–34. https://doi.org/10.1007/s13300-017-0299-7.
Buzzetti R, Pozzilli P, Frederich R, Iqbal N, Hirshberg B. Saxagliptin improves glycaemic control and C-peptide secretion in latent autoimmune diabetes in adults (LADA). Diabetes Metab Res Rev. 2016;32(3):289–96. https://doi.org/10.1002/dmrr.2717.
Cabrera-Rode E, Perich P, Diaz-Horta O, et al. Slowly progressing type 1 diabetes: persistence of islet cell autoantibodies is related to glibenclamide treatment. Autoimmunity. 2002;35(7):469–74. https://doi.org/10.1080/0891693021000050574.
Davis TM, Wright AD, Mehta ZM, et al. Islet autoantibodies in clinically diagnosed type 2 diabetes: prevalence and relationship with metabolic control (UKPDS 70). Diabetologia. 2005;48(4):695–702. https://doi.org/10.1007/s00125-005-1690-x.
Hals IK, Fiskvik Fleiner H, Riemers N, et al. Investigating optimal β-cell-preserving treatment in latent autoimmune diabetes in adults: results from a 21-month randomized trial. Diabetes Obes Metab. 2019;21(10):2219–27. https://doi.org/10.1111/dom.13797.
Johansen OE, Boehm BO, Grill V, et al. C-peptide levels in latent autoimmune diabetes in adults treated with linagliptin versus glimepiride: exploratory results from a 2-year double-blind, randomized, controlled study. Diabetes Care. 2014;37(1):e11–2. https://doi.org/10.2337/dc13-1523.
Kobayashi T, Nakanishi K, Murase T, Kosaka K. Small doses of subcutaneous insulin as a strategy for preventing slowly progressive beta-cell failure in islet cell antibody-positive patients with clinical features of NIDDM. Diabetes. 1996;45(5):622–6. https://doi.org/10.2337/diab.45.5.622.
Yang L, Liang H, Liu X, et al. Islet Function and insulin sensitivity in latent autoimmune diabetes in adults taking sitagliptin: a randomized trial. J Clin Endocrinol Metab. 2021;106(4):e1529–41. https://doi.org/10.1210/clinem/dgab026.
Zhou Z, Li X, Huang G, et al. Rosiglitazone combined with insulin preserves islet beta cell function in adult-onset latent autoimmune diabetes (LADA). Diabetes Metab Res Rev. 2005;21(2):203–8. https://doi.org/10.1002/dmrr.503.
Foley JE, Jordan J. Weight neutrality with the DPP-4 inhibitor, vildagliptin: mechanistic basis and clinical experience. Vasc Health Risk Manag. 2010;9(6):541–8. https://doi.org/10.2147/vhrm.s10952.
Aschner P. Insulin therapy in type 2 diabetes. Am J Ther. 2020;27(1):e79–90. https://doi.org/10.1097/MJT.0000000000001088.
Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2021;44(11):2589–625. https://doi.org/10.2337/dci21-0043.
Maruyama T, Tanaka S, Shimada A, et al. Insulin intervention in slowly progressive insulin-dependent (type 1) diabetes mellitus. J Clin Endocrinol Metab. 2008;93(6):2115–21. https://doi.org/10.1210/jc.2007-2267.
Maddaloni E, Bolli GB, Frier BM, et al. C-peptide determination in the diagnosis of type of diabetes and its management: a clinical perspective. Diabetes Obes Metab. 2022;24(10):1912–26. https://doi.org/10.1111/dom.14785.
Zheng H, Sigal RJ, Coyle D, et al. Comparative efficacy and safety of antihyperglycemic drug classes for patients with type 2 diabetes following failure with metformin monotherapy: a systematic review and network meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2022;38(4):e3515. https://doi.org/10.1002/dmrr.3515.
Takahashi A, Nagashima K, Hamasaki A, et al. Sulfonylurea and glinide reduce insulin content, functional expression of K(ATP) channels, and accelerate apoptotic beta-cell death in the chronic phase. Diabetes Res Clin Pract. 2007;77(3):343–50. https://doi.org/10.1016/j.diabres.2006.12.021.
Nanjan MJ, Mohammed M, Prashantha Kumar BR, Chandrasekar MJN. Thiazolidinediones as antidiabetic agents: a critical review. Bioorg Chem. 2018;77:548–67. https://doi.org/10.1016/j.bioorg.2018.02.009.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705. https://doi.org/10.1016/S0140-6736(06)69705-5.
Thomas MK, Nikooienejad A, Bray R, et al. Dual GIP and GLP-1 receptor agonist tirzepatide improves beta-cell function and insulin sensitivity in type 2 diabetes. J Clin Endocrinol Metab. 2021;106(2):388–96. https://doi.org/10.1210/clinem/dgaa863.
Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2). Diabetes Care. 2015;38(12):2241–9. https://doi.org/10.2337/dc14-1625.
Blonde L, Jendle J, Gross J, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet. 2015;385(9982):2057–66. https://doi.org/10.1016/S0140-6736(15)60936-9.
Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37(8):2149–58. https://doi.org/10.2337/dc13-2761. (Erratum in: Diabetes Care. 2015;38(3):538).
Weinstock RS, Guerci B, Umpierrez G, Nauck MA, Skrivanek Z, Milicevic Z. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes Metab. 2015;17(9):849–58. https://doi.org/10.1111/dom.12479.
von Herrath M, Bain SC, Bode B, et al. Anti-interleukin-21 antibody and liraglutide for the preservation of β-cell function in adults with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2021;9(4):212–24. https://doi.org/10.1016/S2213-8587(21)00019-X.
Wei Z, Yoshihara E, He N, et al. Vitamin D switches BAF complexes to protect β cells. Cell. 2018;173(5):1135-1149.e15. https://doi.org/10.1016/j.cell.2018.04.013.
Zhang Z, Yan X, Wu C, et al. Adding vitamin D3 to the dipeptidyl peptidase-4 inhibitor saxagliptin has the potential to protect β-cell function in LADA patients: a 1-year pilot study. Diabetes Metab Res Rev. 2020;36(5):e3298. https://doi.org/10.1002/dmrr.3298.
Cardoso-Sánchez LI, Gómez-Díaz RA, Wacher NH. Vitamin D intake associates with insulin resistance in type 2 diabetes, but not in latent autoimmune diabetes in adults. Nutr Res. 2015;35(8):689–99. https://doi.org/10.1016/j.nutres.2015.05.019.
Pinheiro MM, Pinheiro FMM, Diniz SN, Fabbri A, Infante M. Combination of vitamin D and dipeptidyl peptidase-4 inhibitors (VIDPP-4i) as an immunomodulation therapy for autoimmune diabetes. Int Immunopharmacol. 2021;95:107518. https://doi.org/10.1016/j.intimp.2021.107518.
Acknowledgements
Author Contributions
Wanqing Wang reviewed data, performed the quality assessment, conducted the meta-analysis and conceived and wrote the manuscript. Fei Huang performed the quality assessment. Chunchao Han resolved inconsistencies of the quality assessment and revised the manuscript critically. All authors approved the final version of the manuscript.
Funding
This study was supported by the Suzhou Gusu Health Talent Program Training Project (No. GSWS2020079) and the Suzhou Science and Technology Bureau Demonstration Project of People's Livelihood Science and Technology (No. SS202006). The Rapid Service Fee was funded by the first author Wanqing Wang.
Medical Writing and Editorial Assistance
The English editorial assistance of the manuscript was provided by the American Journal Experts (AJE) team, and we express our gratitude for their help. The English editorial assistance was funded by the first author Wanqing Wang.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
Ethics committee approval was not required for this network meta-analysis as it entailed the analysis of existing data from previously published literature. A network meta-analysis does not entail direct contact with human participants or collection of new data. Instead, it involves a systematic statistical analysis of data that have already been collected and reported in previously published literature. Therefore, ethics committee permission was deemed unnecessary for this study. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no competing interests to declare that are relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wang, W., Huang, F. & Han, C. Efficacy of Regimens in the Treatment of Latent Autoimmune Diabetes in Adults: A Network Meta-analysis. Diabetes Ther 14, 1723–1752 (2023). https://doi.org/10.1007/s13300-023-01459-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01459-5