Abstract
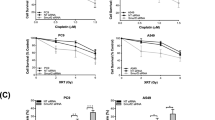
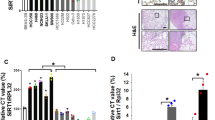
Lung cancer is one of the leading causes of cancer-related death in developed countries. Despite decades of intensive efforts to comate this malignant disease, the prognosis of lung cancer remains unfavorable and is especially poor in advanced non-small cell lung cancer (NSCLC). Accumulating evidence indicate that one of the main causes of the poor outcome in NSCLC treatment is the innate resistance of NSCLC patients to anticancer drugs. However, the mechanism underling NSCLC development and drug resistance is not fully understood. Here we show that the mitochondrial class III NAD+-dependent deacetylase SIRT5 is overexpressed in human NSCLC and high expression of SIRT5 predicts poor survival. SIRT5 knockdown represses lung cancer cell growth and transformation in vitro and in vivo. Furthermore, we find that SIRT5 knockdown makes lung cancer cells more sensitive to drug (cis-diamminedichloroplatinum [CDDP], 5-fluorouracil [5-FU] or bleomycin) treatment in vitro and in vivo. Mechanically, we identify Nrf2, which is a core transcription factor for lung cancer growth and drug resistance, as a target of SIRT5. SIRT5 mRNA level is positively correlated with the expression of Nrf2 in lung cancer tissues and SIRT5 knockdown reduces the expression of Nrf2 and its downstream drug-resistance genes. Taken together, our findings implicate that SIRT5 as a protein responsible for growth and drug resistance in human NSCLC, and SIRT5 may serve as a potential prognostic factor and drug target for intervention.




Similar content being viewed by others
References
Nadkar A, Pungaliya C, Drake K, Zajac E, Singhal SS, Awasthi S. Therapeutic resistance in lung cancer. Expert Opin Drug Metab Toxicol. 2006;2:753–77.
Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–207.
Stępkowski TM, Kruszewski MK. Molecular cross-talk between the NRF2/KEAP1 signaling pathway, autophagy, and apoptosis. Free Radic Biol Med. 2011;50:1186–95.
Zhang DD. The Nrf2-Keap1-ARE signaling pathway: the regulation and dual function of Nrf2 in cancer. Antioxid Redox Signal. 2010;13:1623–6.
Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, et al. Dysfunctional KEAP1–NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420.
Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–9.
Homma S, Ishii Y, Morishima Y, Yamadori T, Matsuno Y, Haraguchi N, et al. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin Cancer Res. 2009;15:3423–32.
Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, et al. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21:689–700.
Singh A, Bodas M, Wakabayashi N, Bunz F, Biswal S. Gain of Nrf2 function in non-small-cell lung cancer cells confers radioresistance. Antioxid Redox Signal. 2010;13:1627–37.
Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–38.
Sun Y, Sun D, Li F, Tian L, Li C, Li L, et al. Downregulation of Sirt1 by antisense oligonucleotides induces apoptosis and enhances radiation sensitization in A549 lung cancer cells. Lung Cancer. 2007;58:21–9.
Jeong SM, Xiao C, Finley LW, Lahusen T, Souza AL, Pierce K, et al. SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell. 2013;23:450–63.
Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137:560–70.
Nakamura Y, Ogura M, Ogura K, Tanaka D, Inagaki N. SIRT5 deacetylates and activates urate oxidase in liver mitochondria of mice. FEBS Lett. 2012;586:4076–81.
Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, et al. Sirt5 Is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–9.
Park J, Chen Y, Tishkoff Daniel X, Peng C, Tan M, Dai L, et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell. 2013;50:919–30.
Xie Z, Dai J, Dai L, Tan M, Cheng Z, Wu Y, et al. Lysine succinylation and lysine malonylation in histones. Mol Cell Proteomics. 2012;11:100–7.
Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol. 2011;7:58–63.
Rardin Matthew J, He W, Nishida Y, Newman John C, Carrico C, Danielson Steven R, et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 2013;18:920–33.
Yn L. Dai D, Lu Q, Fei M, Li M, Wu X. Sirt2 suppresses glioma cell growth through targeting NF-κB–miR-21 axis. Biochem Biophys Res Commun. 2013;441:661–7.
Chen Z, Ye X, Tang N, Shen S, Li Z, Niu X, et al. The histone acetylranseferase hMOF acetylates Nrf2 and regulates anti-drug responses in human non-small cell lung cancer. Br J Pharmacol. 2014;171:3196–211.
Matsuyama W, Nakagawa M, Wakimoto J, Hirotsu Y, Kawabata M, Osame M. Mitochondrial DNA mutation correlates with stage progression and prognosis in non-small cell lung cancer. Hum Mutat. 2003;21:441–3.
Fliss MS, Usadel H, Caballero OL, Wu L, Buta MR, Eleff SM, et al. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science. 2000;287:2017–9.
Hosgood HD, Liu C-S, Rothman N, Weinstein SJ, Bonner MR, Shen M, et al. Mitochondrial DNA copy number and lung cancer risk in a prospective cohort study. Carcinogenesis. 2010;31:847–9.
Ferreira CG, Span SW, Peters GJ, Kruyt FAE, Giaccone G. Chemotherapy triggers apoptosis in a caspase-8-dependent and mitochondria-controlled manner in the non-small cell lung cancer cell line NCI-H460. Cancer Res. 2000;60:7133–41.
Kang MH, Reynolds CP. Bcl-2 Inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–32.
Nakamura Y, Ogura M, Tanaka D, Inagaki N. Localization of mouse mitochondrial SIRT proteins: Shift of SIRT3 to nucleus by co-expression with SIRT5. Biochem Biophys Res Commun. 2008;366:174–9.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 100 kb)
Rights and permissions
About this article
Cite this article
Lu, W., Zuo, Y., Feng, Y. et al. SIRT5 facilitates cancer cell growth and drug resistance in non-small cell lung cancer. Tumor Biol. 35, 10699–10705 (2014). https://doi.org/10.1007/s13277-014-2372-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2372-4