Abstract
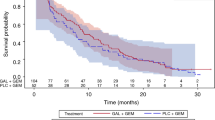
Advanced pancreatic cancer patients have poor prognosis and scarcely respond to conventional therapies. Clinical trials support the use of molecular-targeted therapy against epidermal growth factor receptor (EGFR) signaling. The objective of the current study was to evaluate the contribution of a monoclonal antibody against EGFR, nimotuzumab, to standard gemcitabine therapy. Patients with unresectable locally advanced or metastatic pancreatic adenocarcinoma were assigned to receive gemcitabine plus nimotuzumab. The primary end point was overall survival, whereas the secondary end points included progression-free survival, objective response, and adverse side effects. A total of 18 eligible patients were accrued between December 2007 and July 2010. The disease control rate, calculated as the sum of complete response, partial response, and stable disease, was 55.6 %. The median overall survival time was 9.29 months (95 % CI, 5.499 to 13.072). The median progression-free survival was 3.71 months (95 % CI, 2.526 to 4.902), and the 1-year survival rate was 38.9 %. Of all the patients, 88.8 % had at least one adverse side effect; however, no grade 4 adverse side effect was reported. Nimotuzumab as a high-purity humanized monoclonal antibody with favorable safety profile, its value in the treatment of pancreatic cancer along with gemcitabine, particularly in the comprehensive treatment of advanced pancreatic cancer, is appealing for further prospective randomized large-scale clinical trials.


Similar content being viewed by others
References
GLOBOCAN. Estimated cancer Incidence, Mortality, Prevalence and Disability-adjusted life years (DALYs) Worldwide in 2008. 2008; http://globocan.iarc.fr/. Accessed 15 March 2013.
Jemal A, Siegel R, Ward E, et al. Cancer statistics 2007. CA Cancer J Clin. 2007;57:43–66.
Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet. 2004;363:1049–57.
O'Sullivan A, Kocher HM. Pancreatic cancer. Br Med J Clin Evid. 2007;11:409–37.
Wray CJ, Ahmad SA, Matthews JB, et al. Surgery for pancreatic cancer: recent controversies and current practice. Gastroenterology. 2005;128:1626–41.
Rosewicz S, Wiedenmann B. Pancreatic carcinoma. Lancet. 1997;349:485–9.
Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13.
Hu J, Zhao G, Wang HX, et al. A meta-analysis of gemcitabine containing chemotherapy for locally advanced and metastatic pancreatic adenocarcinoma. J Hematol Oncol. 2011;4:11.
Strimpakos AS, Syrigos KN, Saif MW. Novel agents in early phase clinical studies on refractory pancreatic cancer. JOP. 2012;13:166–8.
Lowery MA, O'Reilly EM. Genomics and pharmacogenomics of pancreatic adenocarcinoma. Pharmacogenomics J. 2012;12:1–9.
Campen CJ, Dragovich T, Baker AF. Management strategies in pancreatic cancer. Am J Health Syst Pharm. 2011;68:573–84.
Yip D, Karapetis C, Strickland A, et al. WITHDRAWN: Chemotherapy and radiotherapy for inoperable advanced pancreatic cancer. Cochrane Database Syst Rev. 2009;4:CD002093.
Kelley RK, Ko AH. Erlotinib in the treatment of advanced pancreatic cancer. Biologics: Targets & Therapy. 2008;2:83–95.
Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol. 2006;33:369–85.
Salomon DS, Brandt R, Ciardiello F, et al. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232.
Troiani T, Martinelli E, Capasso A, et al. Targeting EGFR in pancreatic cancer treatment. Curr Drug Targets. 2012;13:802–10.
Heinemann V, Haas M, Boeck S. Systemic treatment of advanced pancreatic cancer. Cancer Treat Rev. 2012;38:843–53.
Naraev BG, Strosberg JR, Halfdanarson TR. Current status and perspectives of targeted therapy in well-differentiated neuroendocrine tumors. Oncology. 2012;83:117–27.
Talavera A, Friemann R, Gomez-Puerta S, et al. Nimotuzumab, an antitumor antibody that targets the epidermal growth factor receptor, blocks ligand binding while permitting the active receptor conformation. Cancer Res. 2009;69:5851–9.
Boland WK, Bebb G. Nimotuzumab: a novel anti-EGFR mono-clonal antibody that retains anti-EGFR activity while minimizing skin toxicity. Expert Opin Biol Ther. 2009;9:1199–206.
Strumberg D, Schultheis B, Scheulen ME, et al. Phase II study of nimotuzumab, a humanized monoclonal anti-epidermal growth factor receptor (EGFR) antibody, in patients with locally advanced or metastatic pancreatic cancer. Invest New Drugs. 2012;30:1138–43.
Strumberg D, Schultheis B, Scheulen ME, et al. Safety, efficacy and pharmacokinetics of nimotuzumab, a humanized monoclonal anti-epidermal growth factor receptor (EGFR) antibody, in patients with locally advanced or metastatic pancreatic cancer. Int J Clin Pharmacol Ther. 2010;48:473–5.
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Eltawil KM, Renfrew PD, Molinari M. Meta-analysis of phase III randomized trials of molecular targeted therapies for advanced pancreatic cancer. HPB (Oxford). 2012;14:260–8.
Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–10.
Rivera F, Vega-Villegas ME, Lopez-Brea MF, et al. Current situation of Panitumumab, Matuzumab, Nimotuzumab and Zalutumumab. Acta Oncol. 2008;47:9–19.
Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared to gemcitabine alone in patients with advanced pancreatic cancer. A phase III trial of the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG). J Clin Oncol. 2005;23:16s.
Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared to gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6.
Faloppi L, Andrikou K, Cascinu S. Cetuximab: still an option in the treatment of pancreatic cancer? Expert Opin Biol Ther. 2013;13:791–801.
Fensterer H, Schade-Brittinger C, Müller HH, et al. Multicenter phase II trial to investigate safety and efficacy of gemcitabine combined with cetuximab as adjuvant therapy in pancreatic cancer (ATIP). Ann Oncol. 2013;24:2576–81.
Cheng YJ, Bai CM, Zhang ZJ. Efficacy of gemcitabine combined with erlotinib in patients with advanced pancreatic cancer. Acta Acad Med Sin. 2010;32:421–3.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Dan Su and Shun-Chang Jiao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Su, D., Jiao, SC., Wang, LJ. et al. Efficacy of nimotuzumab plus gemcitabine usage as first-line treatment in patients with advanced pancreatic cancer. Tumor Biol. 35, 2313–2318 (2014). https://doi.org/10.1007/s13277-013-1306-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1306-x