Abstract
Background
Sweet osmanthus (Osmanthus fragrans) is an ornamental evergreen tree species in China, whose flowers are sensitive to ethylene. The synthesis of ethylene is controlled by key enzymes and restriction enzymes, 1-aminocyclopropane-1-carboxylic acid synthase (ACS) and 1-aminocyclopropane-1-carboxylic acid oxidase (ACO), which are encoded by multigene families. However, the key synthase responsible for ethylene regulation in O. fragrans is still unknown.
Objective
This study aims to screen the key ethylene synthase genes of sweet osmanthus flowers in response to ethylene regulation.
Methods
In this study, we used the ACO and ACS sequences of Arabidopsis thaliana to search for homologous genes in the O. fragrans petal transcriptome database. These genes were also analyzed bioinformatically. Finally, the expression levels of O. fragrans were compared before and after senescence, as well as after ethephon and silver nitrate treatments.
Results
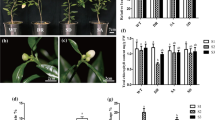
The results showed that there are five ACO genes and one ACS gene in O. fragrans transcriptome database, and the phylogenetic tree revealed that the proteins encoded by these genes had high homology to the ACS and ACO proteins in plants. Sequence alignment shows that the OfACO1-5 proteins have the 2OG-Fe(II) oxygenase domain, while OfACS1 contains seven conserved domains, as well as conserved amino acids in transaminases and glutamate residues related to substrate specificity. Expression analysis revealed that the expression levels of OfACS1 and OfACO1-5 were significantly higher at the early senescence stage compared to the full flowering stage. The transcripts of the OfACS1, OfACO2, and OfACO5 genes were upregulated by treatment with ethephon. However, out of these three genes, only OfACO2 was significantly downregulated by treatment with AgNO3.
Conclusion
Our study found that OfACO2 is an important synthase gene in response to ethylene regulation in sweet osmanthus, which would provide valuable data for further investigation into the mechanisms of ethylene-induced senescence in sweet osmanthus flowers.






Similar content being viewed by others
References
Adams DO, Yang SF (1979) Ethylene biosynthesis: identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. PNAS 76:170–174
Baharudin NF, Osman NI (2023) Plant development, stress responses and secondary metabolism under ethylene regulation. Plant Stress 7:100146
Barry CS, Grierson D, Llop-Tous MI (2000) The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol 123:979–986
Bidonde S, Ferrer MA, Zegzouti H, Ramassamy S, Latche A, Pech JC, Hamilton AJ, Grierson D, Bouzayen M (1998) Expression and characterization of three tomato 1-aminocyclopropane-1-carboxylate oxidase cDNAs in yeast. Eur J Biochem 253:20–26
Binder BM (2020) Ethylene signaling in plants. J Biol Chem 295:7710–7725
Briesemeister S, Rahnenführer J, Kohlbacher O (2010) YLoc—an interpretable web server for predicting subcellular localization. NAR 38:W497–502
Chen H, Zeng X, Yang J, Cai X, Shi Y, Zheng R, Wang Z, Liu J, Yi X, Xiao S et al (2021) Whole-genome resequencing of Osmanthus fragrans provides insights into flower color evolution. Hortic Res 8:98
Cheng Z, Guangwen Z (2000) Physiological and biochemical changes in flower senescence of Osmanthus fragrans Lour. Acta Horticulturae Sinica 27:356–360
Ding W, Ouyang Q, Li Y, Shi T, Li L, Yang X, Ji K, Wang L, Yue Y (2020) Genome-wide investigation of WRKY transcription factors in sweet osmanthus and their potential regulation of aroma synthesis. Tree Physiol 40:557–572
Doan A, Ylmaz G, Erkan M, Baktr I (2013) Effects of sucrose and silver nitrate on the vase life of cut Ranunculus asiaticus L. Acta Hort 1002:341–348
Hayashi T, Teruya T, Chaleckis R, Morigasaki S, Yanagida M (2018) S-adenosylmethionine synthetase is required for cell growth, maintenance of G0 phase, and termination of quiescence in fission yeast. Iscience 5:38–51
Houben M, Van de Poel B (2019) 1-Aminocyclopropane-1-carboxylic acid oxidase (ACO): the enzyme that makes the plant hormone ethylene. Front Plant Sci 10:695
Husain T, Fatima A, Suhel M, Singh S, Sharma A, Prasad SM, Singh VP (2020) A brief appraisal of ethylene signaling under abiotic stress in plants. Plant Signal Behav 15:1782051
Imsabai W, Ketsa S, van Doorn WG (2010) Role of ethylene in the lack of floral opening and in petal blackening of cut lotus (Nelumbo nucifera) flowers. Postharvest Biol Tec 58:57–64
Iqbal N, Khan NA, Ferrante A, Trivellini A, Francini A, Khan M (2017) Ethylene role in plant growth, development and senescence: interaction with other phytohormones. Front Plant Sci 8:475
Jian G, Dong, Woo T, Kim W, Yip K (1991) Cloning of a cDNA encoding 1-aminocyclopropane-1-carboxylate synthase and expression of its mRNA in ripening apple fruit. Planta 185:38–45
John P (1991) How plant molecular biologists revealed a surprising relationship between two enzymes, which took an enzyme out of a membrane where it was not located, and put it into the soluble phase where it could be studied. Plant Mol Biol 9:192–194
Kanojia A, Xu X, Dijkwel PP (2023) Ethylene as a plant aging modulator, 6th edn. Academic Press, Cambridge, pp 73–87
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Lacey RF, Binder BM (2014) How plants sense ethylene gas — the ethylene receptors. J Inorg Biochem 133:58–62
Lee HY, Chen YC, Kieber JJ, Yoon GM (2017) Regulation of the turnover of ACC synthases by phytohormones and heterodimerization in Arabidopsis. Plant J 91:491–504
Liu CY, Lu RH, Li J, Zhao AC, Wang XL, Diane U, Wang XH, Wang CH, Yu YS, Han SM et al (2014) Characterization and expression profiles of MaACS and MaACO genes from mulberry (Morus alba L). J Zhejiang Univ Sci B 15:611–623
Ma N, Cai L, Lu W, Tan H, Gao J (2005) Exogenous ethylene influences flower opening of cut roses (Rosa hybrida) by regulating the genes encoding ethylene biosynthesis enzymes. Sci China C Life Sci 48:434–444
Marić S, Lukić M, Flachowsky H (2014) Allelic polymorphism and inheritance of MdACS1 and MdACO1 genes in apple (Malus × domestica Borkh). Plant Breeding 133:108–114
Mirica LM, Klinman JP (2008) The nature of O2 activation by the ethylene-forming enzyme 1-aminocyclopropane-1-carboxylic acid oxidase. Proc Natl Acad Sci U S A 105:1814–1819
Momonoi K, Shoji K, Yoshida K (2007) Cloning and characterization of ACC oxidase genes from tulip. Plant Biotechnol 24:241–246
Moon J, Park YJ, Son SH, Roh J, Youn JH, Kim SY, Kim SK (2020) Brassinosteroids signaling via BZR1 down-regulates expression of ACC oxidase 4 to control growth of Arabidopsis thaliana seedlings. Plant Signal Behav 15:1734333
Nair SA, Singh V, Sharma T (2003) Effect of chemical preservatives on enhancing vase-life of gerbera flowers. J Trop Agric 41:56–58
Norikoshi R, Niki T, Ichimura K (2022) Differential regulation of two 1-aminocyclopropane-1-carboxylate oxidase (ACO) genes, including the additionally cloned DcACO2, during senescence in carnation flowers. Postharvest Biol Tec 183:111752
Park CH, Roh J, Youn JH, Son SH, Kim SK (2018) Arabidopsis ACC oxidase 1 coordinated by multiple signals mediates ethylene biosynthesis and is involved in root development. Mol Cells 41:923–932
Pattyn J, Vaughan-Hirsch J, Van de Poel B (2021) The regulation of ethylene biosynthesis: a complex multilevel control circuitry. New Phytol 229:770–782
Poel B, Straeten D (2014) 1-Aminocyclopropane-1-carboxylic acid (ACC) in plants: more than just theprecursor of ethylene. Front Plant Sci 5:640
Polko JK, Kieber JJ (2019) 1-Aminocyclopropane 1-carboxylic acid and its emerging role as an ethylene-independent growth regulator. Front Plant Sci 10:1602
Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, Forslund K, Ceric G, Clements J (2004) The pfam protein families database. Nucleic Acids Res 32:D138–D141
Reid MS, Fujino DW, Hoffman NE, Whitehead CS (1984) 1-Aminocyclopropane-l-carboxylic acid (ACC)—the transmitted stimulus in pollinated flowers? J Plant Growth Regul 3:189–196
Rottmann WH, Peter GF, Oeller PW, Keller JA, Shen NF, Nagy BP, Taylor LP, Campbell AD, Theologis A (1991) 1-Aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senescence. J Mol Biol 222:937–961
Ruduś I, Sasiak M, Kępczyński J (2012) Regulation of ethylene biosynthesis at the level of 1-aminocyclopropane-1-carboxylate oxidase (ACO) gene. Acta Physiol Plant 35:295–307
Sato T, Theologis A (1989) Cloning the mRNA encoding 1-aminocyclopropane-1-carboxylate synthase, the key enzyme for ethylene biosynthesis in plants. PNAS 86:6621–6625
Sebastià CH, Hardin SC, Clouse SD, Kieber JJ, Huber SC (2004) Identification of a new motif for CDPK phosphorylation in vitro that suggests ACC synthase may be a CDPK substrate. Arch Biochem Biophys 428:81–91
Shi LS, Liu JP (2016) Molecular cloning and expression analysis of an 1-aminocyclopropane-1-carboxylate synthase gene from Oncidium Gower Ramsey. Biochem Bioph Res Co 469:203–209
Shibuya K, Yoshioka T, Hashiba T, Sato S (2000) Role of the gynoecium in natural senescence of carnation (Dianthus caryophyllus L.) flowers. J Exp Bot 51:2067–2073
Tanase K, Otsu S, Satoh S, Onozaki T (2013) Expression and Regulation of Senescence-related Genes in Carnation Flowers with Low Ethylene Production during Senescence. J Jpn Soc Hort Sci 82(2):179–187
Tang X, Gomes AMTR, Woodson BWR (1994) Pistil-specific and ethylene-regulated expression of 1-aminocyclopropane-1-carboxylate oxidase genes in petunia flowers. Plant Cell 6:1227–1239
Tatsuki M (2001) Phosphorylation of tomato 1-aminocyclopropane-1-carboxylic acid synthase, LE-ACS2, at the C-terminal region. J Biol Chem 276:28051–28057
Thanomchit K, Imsabai W, Burns P, McAtee PA, Schaffer RJ, Allan AC, Ketsa S (2022) Differential expression of ethylene biosynthetic and receptor genes in pollination-induced senescence of Dendrobium florets. Plant Physiol Bioch 188:38–46
Wang KL, Li H, Ecker JR (2002) Ethylene biosynthesis and signaling networks. Plant Cell 14:S131–S151
Wei H, Xue Y, Chen P, Yang Y (2021) Genome-wide identification and functional investigation of 1-aminocyclopropane-1-carboxylic acid oxidase (ACO) genes in cotton. Plants 10:1699
Wu L, Liu J, Huang W, Wang Y, Chen Q, Lu B (2022) Exploration of Osmanthus fragrans Lour.‘s composition, nutraceutical functions and applications. Food Chem 377:131853
Xiaoyan T, Cuiping Y, Jin H, Bingyu Y, Xiaoxiao G, Yufen Z, Yingwen P, Jinping L (2015) Cloning and expression analysis of OnACO2 gene from Oncidium Gower Ramsey. Mol Plant Breed 13:1602–1610
Yang H (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Phys 35:89–155
Yin J, Chang X, Kasuga T, Bui M, Reid MS, Jiang CZ (2015) A basic helix-loop-helix transcription factor, PhFBH4, regulates flower senescence by modulating ethylene biosynthesis pathway in petunia. Hortic Res 2:15059
Zhang C, Wang Y, Fu J, Bao Z, Zhao H (2016) Transcriptomic analysis and carotenogenic gene expression related to petal coloration in Osmanthus fragrans ‘Yanhong Gui.’ Trees 30:1207–1223
Zhao D, Tao J, Zhou C, Wang S (2014) Expression, cloning and characterization of ACC synthase and ACC oxidase genes in Paeonia lactiflora. Int J Agric Biol 16:777–782
Zhou Y, Wang CY, Cheng ZW (2008) Effects of exogenous ethylene and ethylene inhibitor on longevity and peatal senescence of sweet osmanthus. Acta Hort 768:487–493
Zhou L, Jia PY, Liu J, Wang WR, Dong L (2009) Effect of ethylene on cut flowers of tree peony ‘Luoyang Hong’ opening and senescence process and endogenous ethylene biosynthesis. Acta Hortic Sin 36:239–244
Zou J-j, Zhou Y, Cai X, Wang C-y (2014) Increase in DNA fragmentation and the role of ethylene and reactive oxygen species in petal senescence of Osmanthus fragrans. Postharvest Biol Tec 93:97–105
Zou J, Cai X, Wang C (2017) The spatial and temporal distribution of programmed cell death (PCD) during petal senescence of Osmanthus fragrans. Acta Hortic 1185:315–324
Zou J-J, Cai X, Yang J, Zeng X, Liu D-X, Huang S, Chen X, Yang Q-Y, Wang C, Chen H (2023) DNA hypomethylation mediates flower opening and senescence in sweet osmanthus through auxin and ethylene responsive pathways. Postharvest Biol Tec 198:112250
Funding
This work was supported by Zhejiang Provincial Natural Science Foundation of China (Grant Nos. LY19C160002), and the Open Fund of Zhejiang Provincial Key Laboratory of Germplasm Innovation and Utilization for Garden Plants.
Author information
Authors and Affiliations
Contributions
CZ and JF conceived the project and designed the experiments. HQ and YC collected datasets and performed the bioinformatics analysis and expression analysis. HQ, CZ and JF wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
There were no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiu, H., Chen, Y., Fu, J. et al. Expression of ethylene biosynthetic genes during flower senescence and in response to ethephon and silver nitrate treatments in Osmanthus fragrans. Genes Genom 46, 399–408 (2024). https://doi.org/10.1007/s13258-023-01489-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-023-01489-0