Abstract
Introduction
Gestational diabetes is defined as the carbohydrate intolerance of variable severity with onset or first recognition during pregnancy. Gestational glucose intolerance (GGI) is used to indicate pregnant women whose 2-h postprandial glucose is > 120 mg/dl and below 140 mg/dl (Diabetes in Pregnancy Study Group of India, DIPSI criteria).
Aim
This study was planned to see whether intervention in GGI group helps to improve feto-maternal outcomes.
Methodology
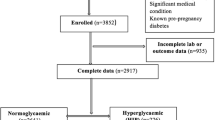
This open-label randomized control trial was conducted in Department of Obstetrics and Gynaecology of King George's Medical University, Lucknow. Inclusion criteria were all the antenatal women attending the antenatal clinic and diagnosed as GGI, and exclusion criteria were overt diabetes.
Results
Total of 1866 antenatal women were screened, and among them, 220 (11.8%) women were diagnosed as gestational diabetes; 412 (22.1%) women were diagnosed as GGI. The mean fasting blood sugars in the women with GGI who had medical nutrition therapy were much lower than the women with GGI who did not have any intervention. The present study showed the women with GGI had higher complications like polyhydramnios, PPROM, foetal growth restriction, macrosomia, preeclampsia, preterm labour and vaginal candidiasis more in the women with GGI as compared to euglycaemic women.
Conclusion
The present study of nutritional intervention in GGI group has shown trend towards lesser complication if we start medical nutrition therapy reflected by delayed development of GDM and less neonatal hypoglycaemia and hyperbilirubinemia.

Similar content being viewed by others
References
Cunningham FG, Leveno KJ, Bloom SL, et al. Chapter No 57. Diabetes mellitus. In: Twickler DM, Mahendroo MS, editors., et al., Williams obstetrics and gynaecology. 24th ed. New York: McGraw-Hill Education; 2014. p. 1136.
Sacks DA, Hadden R, Maresh M, et al. HAPO study cooperative research group: frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel recommended criteria. Diabetes Care. 2012;35:526–8.
Jhalani M, Gurnani V, Agnani M, et al. Diagnosis & management of gestational diabetes mellitus. Technical and operational guidelines. February 2018, p. 21–5. MOHFW. www.mohfw.gov.in. Accessed 16 Aug 2018.
Seshiah V, Das AK, Balaji V, et al. Gestational diabetes mellitus—guidelines. J Assoc Physicians India. 2006;54:622–8.
American Diabetes Association. Standards of medical care in diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1–106.
Bonomo M, Gandini ML, Mastropasqua A, et al. Which cutoff level should be used in screening for glucose intolerance in pregnancy? Definition of Screening Methods for Gestational Diabetes Study Group of the Lombardy Section of the Italian Society of Diabetology. Am J Obstet Gynecol. 1998;179:179–85.
Yang X, Hage HB, Zhang H, et al. Women with impaired glucose tolerance during pregnancy have significantly poor pregnancy outcomes. Diabetes Care. 2002;25:1619–24.
Jensen DM, Korsholm L, Ovesen P, et al. Adverse pregnancy outcome in women with mild glucose intolerance: is there a clinically meaningful threshold value for glucose? Acta Obstet Gynecol Scand. 2008;87(1):59–62.
Mirzamoradi M, Bakhtiyari M, Kimiaee P, et al. Investigating the effects of treatment based on single high blood glucose in gestational diabetes screening on maternal and neonatal complications. Arch Gynecol Obstet. 2015;292(3):687–95.
Acknowledgements
We acknowledge the contribution of pregnant women visiting us.
Author information
Authors and Affiliations
Contributions
VD laid down the hypothesis and concept of the study. AS and NK carried out the recruitment and study documentation. AA helped in literature search. SA helped in follow-up of patients, and AP helped in data analysis.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest among the authors. There is no financial relationship with any organizations.
Ethical Approval
All procedures followed were in accordance with the ethical standards of the responsible institutional committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed Consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Namrata Kumar is an Associate Professor.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, N., Das, V., Srivastava, A. et al. Can Medical Nutrition Therapy Affect Feto-Maternal Outcomes in Gestational Glucose Intolerance: An Open-Label Pilot Randomized Control Trial in World’s Diabetes Capital. J Obstet Gynecol India 73, 208–213 (2023). https://doi.org/10.1007/s13224-022-01722-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13224-022-01722-y