Abstract
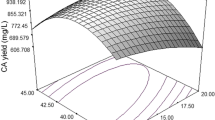
The purpose of this article is to use statistical Plackett–Burman and Box–Wilson response surface methodology to optimize the medium components and, thus, improve chitinase production by Streptomyces griseorubens C9. This strain was previously isolated and identified from a semi-arid soil of Laghouat region (Algeria). First, syrup of date, colloidal chitin, yeast extract and K2HPO4, KH2PO4 were proved to have significant effects on chitinase activity using the Plackett–Burman design. Then, an optimal medium was obtained by a Box–Wilson factorial design of response surface methodology in liquid culture. Maximum chitinase production was predicted in medium containing 2% colloidal chitin, 0.47% syrup of date, 0.25 g/l yeast extract and 1.81 g/l K2HPO4, KH2PO4 using response surface plots of the STATISTICA software v.12.0.



Similar content being viewed by others
References
Adinarayana K, Ellaiah P (2002) Response surface optimization of the critical medium components for the production of alkaline protease by a newly isolated Bacillus sp. J Pharm Pharm Sci 5:272–278
Andronopoulou E, Vorgias CE (2004) Multiple components and induction mechanism of the chitinolytic system of the hyperthermophilic archaeon Thermococcus chitonophagus. Appl Microbiol Biotechnol 65:694–702. doi:10.1007/s00253-004-1640-4
Attwood MM, Zola H (1967) The association between chitin and protein in some chitinous tissues. Comp Biochem Physiol 20:993–998. doi:10.1016/0010-406X(67)90069-2
Box GE, Wilson KB (1951) On the experimental attainment of optimum conditions. J R Stat Soc Ser B 13:1–45
Box GEP (1952) Multi-factor designs of first order. Biometrika 39:49–57. doi:10.1093/biomet/39.1-2.49
Ehrlich H, Krautter M, Hanke T et al (2007a) First evidence of the presence of chitin in skeletons of marine sponges. Part II. Glass sponges (Hexactinellida: Porifera). J Exp Zool B 308:473–483. doi:10.1002/jez.b.21174
Ehrlich H, Maldonado M, Spindler K et al (2007b) First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (demospongia: Porifera). J Exp Zool B 308B:347–356. doi:10.1002/jez.b.21156
Elibol M (2004) Optimization of medium composition for actinorhodin production by Streptomyces coelicolor A3 (2) with response surface methodology. Process Biochem 39:1057–1062
Ergun M, Mutlu SF (2000) Application of a statistical technique to the production of ethanol from sugar beet molasses by Saccharomyces cerevisiae. Bioresour Technol 73:251–255
Fenice M, Leuba J-L, Federici F (1998) Chitinolytic enzyme activity of Penicillium janthinellum P9 in bench-top bioreactor. J Ferment Bioeng 86:620–623. doi:10.1016/S0922-338X(99)80020-8
Funkhouser JD, Aronson NN (2007) Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol Biol 7:96. doi:10.1186/1471-2148-7-96
Gheshlaghi R, Scharer JM, Moo-Young M, Douglas PL (2005) Medium optimization for hen egg white lysozyme production by recombinant Aspergillus niger using statistical methods. Biotechnol Bioeng 90:754–760
Gohel V, Naseby DC (2007) Thermalstabilization of chitinolytic enzymes of Pantoea dispersa. doi: 10.1016/j.bej.2007.01.009
Gong X, Chen F (1997) Optimization of culture medium for growth of Haematococcus pluvialis. J Appl Phycol 9:437–444
Gooday GW (1990) The ecology of chitin degradation. In: Marshall KC (ed) Advances in microbial ecology. Springer, New York, pp 387–430
Han Y, Yang B, Zhang F et al (2009) Characterization of antifungal chitinase from marine Streptomyces sp. DA11 associated with South China Sea sponge Craniella australiensis. Mar Biotechnol 11:132–140. doi:10.1007/s10126-008-9126-5
He J, Zhen Q, Qiu N et al (2009) Medium optimization for the production of a novel bioflocculant from Halomonas sp. V3a′ using response surface methodology. Bioresour Technol 100:5922–5927
Hsu SC, Lockwood JL (1975) Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. Appl Microbiol 29:422–426
Huang X, Wang Y, Cui Y, Hua X (2010) Optimization of antifungal effect of Surfactin and Iturin to Penicillium notatum in syrup of peach by RSM. Int J Pept Res Ther 16:63–69
Kaiser K, Benner R (2008) Major bacterial contribution to the ocean reservoir of detrital organic carbon and nitrogen. Limnol Oceanogr 53:99–112. doi:10.4319/lo.2008.53.1.0099
Kamel BS (1979) Dates as a potential substrate for single cell protein production. Enzym Microb Technol 1:180–182
Khan MA (2010) Optimization of culture media for enhanced chitinase production from a novel strain of Stenotrophomonas maltophilia using response surface methodology. J Microbiol Biotechnol 20:1597–1602. doi:10.4014/jmb.0909.09040
Merzendorfer H, Zimoch L (2003) Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J Exp Biol 206:4393–4412
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. doi:10.1021/ac60147a030
Monreal J, Reese ET (1969) The chitinase of Serratia marcescens. Can J Microbiol 15:689–696
Montgomery DC (2008) Design and analysis of experiments. Wiley, New York
Nancib N, Nancib A, Boudjelal A et al (2001) The effect of supplementation by different nitrogen sources on the production of lactic acid from date juice by Lactobacillus casei subsp. rhamnosus. Bioresour Technol 78:149–153
Narayana KJP, Vijayalakshmi M (2009) Chitinase production by streptomyces sp. ANU 6277. Braz J Microbiol. doi:10.1590/S1517-83822009000400002
Nawani NN, Kapadnis BP (2005) Optimization of chitinase production using statistics based experimental designs. Process Biochem 40:651–660
Nielsen MN, Sørensen J (1999) Chitinolytic activity of Pseudomonas fluorescens isolates from barley and sugar beet rhizosphere. FEMS Microbiol Ecol 30:217–227
Pan CM, Fan YT, Xing Y et al (2008) Statistical optimization of process parameters on biohydrogen production from glucose by Clostridium sp. Fanp2. Bioresour Technol 99:3146–3154
Patel B, Gohel V, Raol B (2007) Statistical optimisation of medium components for chitinase production byPaenibacillus sabina strain JD2. Ann Microbiol 57:589–597
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 33:305. doi:10.2307/2332195
Radford A (1991) Methods in yeast genetics — A laboratory course manual by M Rose, F Winston and P Hieter. Biochem Educ 19:101–102. doi:10.1016/0307-4412(91)90039-B
Rao KJ, Kim C-H, Rhee S-K (2000) Statistical optimization of medium for the production of recombinant hirudin from Saccharomyces cerevisiae using response surface methodology. Process Biochem 35:639–647
Reynolds DM (1954) Exocellular chitinase from a Streptomyces sp. Microbiology 11:150–159
Roukas T, Kotzekidou P (1997) Pretreatment of date syrup to increase citric acid production. Enzym Microb Technol 21:273–276
Schaefer J, Kramer KJ, Garbow JR et al (1987) Aromatic cross-links in insect cuticle: detection by solid-state 13C and 15N NMR. Science 235:1200–1204
Sherief AA, El-Sawah MMA, El-Naby MAA (1991) Some properties of chitinase produced by a potent Aspergillus carneus strain. Appl Microbiol Biotechnol 35:228–230
Singh AK, Mehta G, Chhatpar HS (2009) Optimization of medium constituents for improved chitinase production by Paenibacillus sp. D1 using statistical approach. Lett Appl Microbiol 49:708–714
St Leger RJ, Cooper RM, Charnley AK (1986) Cuticle-degrading enzymes of entomopathogenic fungi: regulation of production of chitinolytic enzymes. Microbiology 132:1509–1517
Treichel H, de Oliveira D, Mazutti MA et al (2010) A review on microbial lipases production. Food Bioprocess Technol 3:182–196
Ulhoa CJ, Peberdy JF (1991) Regulation of chitinase synthesis in Trichoderma harzianum. Microbiology 137:2163–2169. doi:10.1099/00221287-137-9-2163
Usui T, Hayashi Y, Nanjo F et al (1987) Transglycosylation reaction of a chitinase purified from Nocardia orientalis. Biochim Biophys Acta Gen Subj 923:302–309
Vaidya R, Vyas P, Chhatpar H (2003) Statistical optimization of medium components for the production of chitinase by Alcaligenes xylosoxydans. Enzym Microb Technol 33:92–96. doi:10.1016/S0141-0229(03)00100-5
Vaidya RJ, Shah IM, Vyas PR, Chhatpar HS (2001) Production of chitinase and its optimization from a novel isolate Alcaligenes xylosoxydans: potential in antifungal biocontrol. World J Microbiol Biotechnol 17:691–696
Wang Z-W, Liu X-L (2008) Medium optimization for antifungal active substances production from a newly isolated Paenibacillus sp. using response surface methodology. Bioresour Technol 99:8245–8251
Whitman WB, Coleman DC, Wiebe WJ (1998) Prokaryotes: the unseen majority. Proc Natl Acad Sci U S A 95:6578–6583
Yu X, Hallett SG, Sheppard J, Watson AK (1997) Application of the Plackett-Burman experimental design to evaluate nutritional requirements for the production of Colletotrichum coccodes spores. Appl Microbiol Biotechnol 47:301–305
Acknowledgements
We thank Prof. A. Boulahrouf, head of applied microbiology team at the engineering microbiology and applications laboratory (Constantine University) for helping us in this work. Thanks are expressed to France Chitin (www.france-chitine.com) for a gift of chitin. We also thank M. Merouane Fateh and M. Mezdour Hichem (Constantine University) for their technical advice. This work was supported by Mentouri Brothers University Constantine, Algeria.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meriem, G., Mahmoud, K. Optimization of chitinase production by a new Streptomyces griseorubens C9 isolate using response surface methodology. Ann Microbiol 67, 175–183 (2017). https://doi.org/10.1007/s13213-016-1249-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-016-1249-8