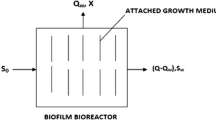
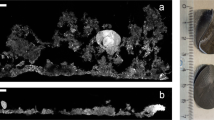
Abstract
The impact of various parameters, such as nutrient, temperature, surface materials and condition and hydrodynamics, on biofilm formation is well studied. Extensive research has focused on the relationship between these parameters and bacterial biofilms, with the aim of gaining an understanding of biofilm behaviour under different growth conditions so that relevant control strategies can be implemented. In such studies, model simulations have been used to qualitative study the behaviour of the biofilms respond to change in parameters. However, little is known about the quantitative study of biofilm behaviour in response to change in these parameters. In previous studies, it was indicated that nutrient concentrations influence biofilm morphology (biomass, structures and thickness) but the concentration levels at which biofilm change in structure and thickness is not mentioned. These observations were based on determining biofilms structure without considering the biomass. Findings that are based on qualitative studies only may be insufficient and not in supportive due to the fact that may be pose many speculations and debates. The biomass, structures and thickness form biofilm morphology, therefore if one part is affected, the other parts may also be affected. It is important to conduct research that will focus on both qualitative and qualitative analysis on the impact of parameters on biofilm formation and growth. The aim of this review is to highlight the importance of conducting parallel research on quantitative and qualitative study on microbial biofilms with respect to biomass, structure and thickness.



Similar content being viewed by others
References
Allison DG (2003) The biofilm matrix. Biofoul 19:139–150
An YH, Dickison R, Doyle RJ (2000) Mechanisms of bacterial adhesion and pathogenesis of implant and tissue infections. In: An YH, Friedman RJ (eds) Handbook of bacterial adhesion: Principles, methods and applications. Humana Press, Totowa, pp 1–27
Apilanez I, Gutierrez A, Diaz M (1998) Effect of surface material on initial biofilm development. Biores Technol 66:225–230
Bhaskar PV, Bhosle NB (2005) Microbial extracellular polymeric substances in marine biogeochemical processes. Curr Sci 88:47–53
Breyers JD, Ratner JP (2004) Bioinspired implant materials befuddle bacteria. ASM News 70:232–237
Bonaventura GD, Piccolomini R, Paludi D, D’Orio V, Vergara A, Conter M, Ianieri A (2008) Influence of temperature on biofilm formation by Listeria monocytogenes on various food-contact surfaces: relationship with motility and cell surface hydrophobicity. J Appl Microbiol 104:1552–1561
Camper AK, Warren LJ, Jason TH (1996) Effect of growth conditions and substratum composition on the persistence of coliforms in mixed-population biofilms. Appl Environ Microbiol 62:4014–4018
Carlen A, Nikdel K, Wennerberg A, Holmberg K, Olsson J (2001) Surface characteristics and in vitro biofilm formation on glass ionomer and composite resin. Biomaterials 22:481–487
Cheng G, Zhang Z, Chen S, Bryers J, Jiang S (2007) Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials 29:4192–4199
Chmielewski RAN, Frank JF (2003) Biofilm Formation and control in food processing facilities. Comprehensive review in food science and food safety. Inst Food Technol 2:22–32
Cloete TE, Westaard D, van Vuuren SJ (2003) Dynamic response of biofilm to pipe surface and fluid velocity. Water Sc iTechnol 47(5):57–59
Coleman DC, O’Donnell MJ, Shore AC, Swan J, Russell RJ (2010) The role of manufacturers in reducing biofilms in dental chair waterlines. J Dent 35:701–711
Costerton JW (1995) Overview of microbial biofilms. J Ind Microbiol 15:137–140
Dignac MF, Urbain V, Rybacki D, Bruchet A, Snidaro D, Scribe P (1998) Chemical description of extracellular polymers: implication on activated sludge floc structure. Water Sci Technol 38:45–53
Donlan RM (2002) Biofilms and device-associated infections. Emerg Infect Dis 7(2):277–281
Dunne WM (2002) Bacterial adhesion: Seen any good biofilms lately? J Clin Microbiol 15:155–166
Dunsmore BC, Jacobsen A, Hall-Stoodley L, Bass CJ, Lappin-Scott HM, Stoodley P (2002) The influence of fluid shear on the structure and material properties of sulphate-reducing bacterial biofilms. J Ind Microbiol Biotechnol 29:347–353
Faille C, Jullien C, Fontaine F, Bellon-Fontaine MN, Slomianny C, Bénézech T (2002) Adhesion of Bacillus spores and Escherichia coli cells to inert surfaces: role of surface hydrophobicity. Can J Microbiol 48:728–738
Fang HHP, Liu H, Zhang T (2002) Characterization of hydrogen producing granular sludge. Biotechnol Bioeng 78:44–52
Flemming HC, Wingender J, Mayer C, Kostgens V, Borchard W (2000) Cohesiveness in biofilm matrix polymers. In: Community structure and cooperation in biofilms. Press Syndicate, Cambridge, p 91
Flemming HC, Neu TR, Wozniak D (2007) The EPS matrix: The “house of biofilms cells”. J Bacteriol 189(22):1–6
Florjanic M, Kristl J (2011) The control of biofilm formation by hydrodynamics of purified water in industrial distribution system. Int J Pharm 405:16–22
Ghannoum M, O’Toole GA (2004) Microbial biofilms. American Soc Microbiol Press, Washington D.C., pp 250–268
Girbal-Neuhauser E (2011) Extracellular polymeric substances diversity of biofilms grown under contrasted environmental conditions. Water Res 45:1529–1538
Horswill AR, Stoodley P, Stewart PS, Parsek MR (2007) The effect of the chemical, biological, and physical environment on quorum sensing in structured microbial communities. Anal Bioanal Chem 387:371–380
Hoyle B (1992) Pseudomonas aeruginosa biofilm as a diffusion barrier to piperacillin. J Ant Agents Chem 36:2054–2056
Jiao Y, Cody GD, Harding AK, Wilmes P, Schrenk M, Wheeler KE, Banfield JF, Thelen MP (2010) Characterization of extracellular polymeric substances from acidophilic microbial biofilms. Appl Environ Microbiol 76(9):2916–2922
Kalmokoff ML, Austin JW, Wan XD, Sanders G, Banerjee S, Farber JM (2001) Adsorption, attachment and biofilm formation among isolates of Listeria monocytogenes using model conditions. J Appl Microbiol 91:725–734
Klahre J, Flemming HC (2000) Monitoring of biofouling in papermill process. Water Res 34(14):3657–3665
Kristensen JB, Meyer RL, Lauren BS, Shipovskov S, Besenbacher F, Poulsen CH (2008) Antifouling enzymes and the biochemistry of marine settlement. J Biotechnol 26:471–481
Kumar A, Prasad R (2006) Biofilms. JK Sci 8:15–17
Li Y, Hao G, Galvani CD, Meng Y, de la Fuente L, Hoch HC, Burr TJ (2007) Type I and type IV pili of Xylella fastidiosa affect twitching motility, biofilm formation and cell- cell aggregation. J Microbiol 153:719–726
Liu H, Fang HP (2002) Extraction of extracellular polymeric substances (EPS) of sludge. J Biotechnol 95:249–256
Liu L, Chu L, Liu Q, Wang C, Xia Y, Peng X (2010) A comparative study on biofilm formation of nontypeable Haemophilus influenzae and Pseudomonas aeruginosa under single culture or co-culture. Afr J Microbiol Res 4(3):180–184
Liu Y, Yang SF, Li Y, Xu H, Qin L, Tay JH (2004) The influence of cell substratum surface hydrophobicities on microbial attachment. J Biotechnol 110:251–256
Mains C (2008) Biofilm control in distribution systems. Natl Environ Serv Center (NESC) 8(2):1–4
Molobela IP, Cloete TE, Beukes M (2010) Protease and amylase enzymes for biofilm removal and degradation of extracellular polymeric substances (EPS) produced by Pseudomonas fluorescens bacteria. Afr J Microbiol Res 4(14):1515–1524
Noguera DR, Okabe S, Picioreanu C (1999) Biofilm modelling: present status and future dicetions. Water Sci Technol 39(7):273–278
O’Toole G, Kaplan HB, Kolter R (2000) Biofilm formation as microbial development. Annu Rev Microbiol 54:49–79
Oh YJ, Jo W (2007) Biofilm formation and local electrostatic force characteristics of Escherichia coli 0157:H7 observed by electrostatic microscopy. Appl Phys Letters 90:143901
Pan X, Liu J, Zhang D, Chen X, Li L, Song W, Yang J (2010) A comparison of five extraction methods for extracellular polymeric substances (EPS) from biofilm by using three-dimensional excitation-emission matrix (3DEEM) fluorescence spectroscopy. Water SA 36(1):111–116
Pei-shi QI, Wen-bin W, Zheng, QI (2008) Effect of shear stress on biofilm morphological characteristics and the secretion of extracellular polymeric substances. School of Municipal & Environmental Engineering. Harbin Institute of Technology. Harbin, pp 3438–3441
Prakash B, Veeregowda BM, Krishnappa G (2003) Biofilms: A survival strategy of bacteria. J Curr Sci 85:9–10
Purevdorj B, Costerton JW, Stoodley P (2002) A influence of hydrodynamics and cell signaling on the structure and behavior of Pseudomonas aeruginosa biofilms. Appl Environ Microbiol 68(9):4457–4464
Rachid S, Ohlsen K, Witte W, Hacker J, Ziebuhr W (2000) Effect of sub inhibitory antibiotic concentrations on polysaccharide intercellular adhesion expression in biofilm forming Staphylococcus epidermidis. J Ant Agents Chem 44:3357–3363
Rao TS (2010) Comparative effect of temperature on biofilm formation in natural and modified marine environment. Aquat Ecol 44:463–478
Ras M, Girbal-Neuhauser E, Paul EM, Sperandio M, Lefebvre D (2008) Protein extraction from activated sludge: An analytical approach. Water Res 42:1867–1878
Rice SA, Koh KS, Queck SY, Labbate M, Lam KW, Kjelleberg S (2005) Biofilm formation and sloughing in Serratia marcescens are controlled by quorum sensing and nutrient cues. J Bacteriol 187(10):3477–85
Rinaudi L, Fujishinge NA, Hirsch AM, Banchio E, Zorreguieta A, Giordano W (2006) Effects of nutritional and environmental conditions on Sinorhizobium meliloti biofilm formation. Res Microbiol 157:867–875
Rochex A, Lebeault JM (2007) Effects of nutrients on biofilm formation and detachment of a Pseudomonas putida strain isolated from a paper machine. Water Res 41:2885–2892
Sauer K, Camper AK (2001) Characterization of phenotypic changes in Pseudomonas putida in response to surface associated growth. J Bacteriol 183:6579–6589
Simoes M, Simoes LC, Vieira MJ (2010) A review of current and emergent biofilm control strategies. Food Sci Technol 43:573–583
Simoes M, Pereira MO, Sillankorva S, Azeredo J, Viera MJ (2007) The effect of hydrodynamic conditions on the phenotype of Pseudomonas fluorescens biofilms. Biofoul 23(3/4):249–258
Smith AW (2005) Biofilms and antibiotic therapy: Is there a role for combating bacterial resistance by the use of novel drug delivery system? J Adv Drug Delivery Review 57:1539–1550
Stoodley P, Dodds I, Boyle JD, Lappin-Scott HM (1999) Influence of hydrodynamics and nutrients on biofilm structure. J Appl Microbiol 85:19–28
Stoodley P, Cargo R, Rupp CJ, Wilson S, Klapper I (2002) Biofilm material properties as related to shear-induced deformation and detachment phenomena. J Ind Microbiol Biotechnol 29:361–367
Sutherland IW (1994) Structure- function relationship in microbial exopolysaccharides. J Biotechnol Adv 12:393–448
Vieira MJ, Melo LF, Pinheiro MM (1993) Biofilm formation: Hydrodynamic effects on internal diffusion and structure. J Bioad Biofilm Res 7(1):67–80
Villa F, Albanese D, Giussani B, Stewart P, Daffonchio D, Cappitelli F (2010) Hindering biofilm formation with zosteric acid. Biofoul 26:739–752
Vu B, Chen M, Russell JC, Ivanova EP (2009) Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 14:2535–2554
Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S, Greenberg EP (2001) Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860–864
Yongqin JY, Cody GD, Harding AK, Wilmes P, Schrenk M, Wheeler KE, Banfield JF, Thelen MP (2010) Characterization of Extracellular Polymeric Substances from acidophilic microbial biofilms. Appl Environ Microbiol 76(9):2916–2922
Zacheus OM, Livanainen EK, Nissinen TK, Lehtola MJ, Martikainen PJ (2000) Bacterial biofilm formation on polyvinyl chloride, polyethylene and stainless steel exposed to ozonated water. Water Res 1:63–70
Zhang T, Fang HP (2001) Quantification of extracellular polymeric substances in biofilms by confocal laser scanning microscopy. J Biotechnol 23:405–409
Acknowledgements
The authors would like to thank the University of South Africa for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Molobela, I.P., Ilunga, F.M. Impact of bacterial biofilms: the importance of quantitative biofilm studies. Ann Microbiol 62, 461–467 (2012). https://doi.org/10.1007/s13213-011-0344-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-011-0344-0