Abstract
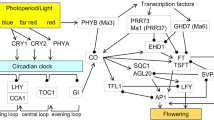
Flowering is a crucial phase for angiosperms to continue their species propagation and is highly regulated. In the current review, flowering in sugarcane and the associated mechanisms are elaborately presented. In sugarcane, flowering has two effects, wherein it is a beneficial factor from the breeder’s perspective and crucial for crop improvement, but commercially, it depletes the sucrose reserves from the stalks; hence, less value is assigned. Different species of Saccharum genus are spread across geographical latitudes, thereby proving their ability to grow in multiple inductive daylengths of different locations according in the habituated zone. In general, sugarcane is termed an intermediate daylength plant with quantitative short-day behaviour as it requires reduction in daylength from 12 h 55 min to 12 h or 12 h 30 min. The prime concern in sugarcane flowering is its erratic flowering nature. The transition to reproductive stage which reverts to vegetative stage if there is any deviation from ambient temperature and light is also an issue. Spatial and temporal gene expression patterns during vegetative to reproductive stage transition and after reverting to vegetative state could possibly reveal how the genetic circuits are being governed. This review will also shed a light on potential roles of genes and/or miRNAs in flowering in sugarcane. Knowledge of transcriptomic background of circadian, photoperiod, and gibberellin pathways in sugarcane will enable us to better understand of variable response in floral development.







Similar content being viewed by others
References
Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. J Sci 309:1052–1056
Ahmed M, Rahman F (2014) Changing paradigms in regulating and deregulating the sugar pricing mechanism in India. Saaransh RKG J Manag 5:77–86
Ahn JH, Miller D, Winter VJ, Banfield MJ, Lee JH, Yoo SY, Henz SR, Brady RL, Weigel D (2006) A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J 25:605–614
Andres F, Coupland G (2012) The genetic basis of flowering responses to seasonal cues. J Nat Rev Genet 13:627–639
Banfield MJ, Barker JJ, Perry AC, Brady RL (1998) Function from structure? The crystal structure of human phosphatidylethanolamine-binding protein suggests a role in membrane signal transduction. J Struct 6:1245–1254
Bao S, Hua C, Shen L, Yu H (2019) New insights into gibberellin signaling in regulating flowering in Arabidopsis. J Integr Plant Biol 62:118–131
Bauer D, Vicziaén A, Kircher S, Nobis T, Nitschke R, Kunkel T, Panigrahi KCS, Aedaem E, Fejes E, Scafer E, Nagy F (2004) Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. J Plant Cell 16:1433–1445
Bell CI, Leigh RA (1996) Differential effects of turgor on sucrose and potassium transport at the tonoplast and plasma membrane of sugar beet storage root tissue. J Plant Cell Environ 19:191–200
Bernier G, Périlleux C (2005) A physiological overview of the genetics of flowering time control. J Plant Biotechnol J 3:3–16
Blázquez MA, Ahn JH, Weigel D (2003) A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nat genet 33(2):168–171
Bradley D, Carpenter R, Copsey L, Vincent C, Rothstein S, Coen E (1996) Control of inflorescence architecture in Antirrhinum. J Nat 379:791–797
Brunkhorst MJ (2001) A preliminary investigation into the effect of plant nutrient levels on sugarcane flowering. Proc S Afr Sugar Technol Assoc 75:143–150
Butterfield MK, D’Hont A, Berding N (2001) The sugarcane genome: a synthesis of current understanding, and lessons for breeding and biotechnology. Proc S Afr Sug Technol Ass 75:1–5
Calderan-Rodrigues MJ, de Barros Dantas LL, Cheavegatti Gianotto A, Caldana C (2021) Applying molecular phenotyping tools to explore sugarcane carbon potential. Front Plant Sci 12:637166. https://doi.org/10.3389/fpls.2021.637166
Capovilla G, Schmid M, Posé D (2015) Control of flowering by ambient temperature. J Exp Bot 66:59–69
Castillejo C, Pelaz S (2008) The balance between CONSTANS and TEMPRANILLO activities determines FT expression to trigger flowering. J Curr Biol 18:1338–1343
Chandra P, Singh IS, Singh SB (2005) Biochemical changes during flowering of sugarcane. J Sugar Tech 7:160–162
Chardon F, Damerval C (2005) Phylogenomic analysis of the PEBP gene family in cereals. J Mol Evol 61:579–590
Cheavegatti-Gianotto A, de Abreu HMC, Arruda P, Bespalhok Filho JC, Burnquist WL, Creste S, di Ciero L, Ferro JA, de Oliveira FAV, de Sousa FT, Grossi-de-Sa M, Guzzo EC, Hoffmann HP, de Andrade LMG, Macedo N, Matsuoka S, de Castro RF, Romano E, da Silva WJ (2011) Sugarcane (Saccharum X officinarum): a reference study for the regulation of genetically modified cultivars in Brazil. J Trop Plant Biol 4:62–89
Clements HF, Awada M (1967) Experiments on the artificial induction of flowering in sugarcane. Proc Int Soc Sug Cane Technol 12:795–812
Coelho CP, Costa Netto AP, Colasanti J, Chalfun-Junior A (2013) A proposed model for the flowering signaling pathway of sugarcane under photoperiodic control. J Genet Mol Res 12:1347–1359
Coelho CP, Minow MA, Chalfun-Júnior A, Colasanti J (2014) Putative sugarcane FT/TFL1 genes delay flowering time and alter reproductive architecture in Arabidopsis. J Front Plant Sci 5:1–12
Colasanti J, Coneva V (2009) Mechanisms of floral induction in grasses: something borrowed, something new. J Plant Physiol 149:56–62
Coleman RE (1969) Physiology of flowering in sugarcane. Proceedings of the international society of sugarcane technologists 13th congress, pp 992–1000
Conti L, Bradley D (2007) TERMINAL FLOWER1 is a mobile signal controlling Arabidopsis architecture. J Plant Cell 19:767–778
Creux N, Harmer S (2019) Circadian rhythms in plants. J Cold Spring Harbor Perspect Biol 11:a034611
Daniels J, Smith P, Paton N, Williams CA (1975) The origin of the genus Saccharum. Sugarcane Breed Newslett 36:24–39
Daniels J, Roach BT (1987) Taxonomy and evolution. In: Sugarcane improvement through breeding
De Lucas M, Daviere JM, Rodríguez-Falcón M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blazquez MA, Prat S (2008) A molecular framework for light and gibberellin control of cell elongation. J Nat 451:480–484
Deng W, Ying H, Helliwell CA, Taylor JM, Peacock WJ, Dennis ES (2011) FLOWERING LOCUS C (FLC) regulates development pathways throughout the life cycle of Arabidopsis. Proc Natl Acad Sci 108:6680–6685
Devlin PF, Kay SA (2000) Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity. J Plant Cell 12:2499–2509
D’Hont A, Grivet L, Feldmann P, Glaszmann JC, Rao S, Berding N (1996) Characterisation of the double genome structure of modern sugarcane cultivars (Saccharum spp.) by molecular cytogenetics. J Mol General Genet MGG 250:405–413
Dlamini PJ (2021) Drought stress tolerance mechanisms and breeding effort in sugarcane: a review of progress and constraints in South Africa. Plant Stress 2:100027
Edwards E, Paxton JG (1979) Effects of photoperiod and temperature on the rate of elongation of sugarcane leaf sheaths. Proc South Afr Sugar Technol Assoc 53:163–164
Endres L, da Cruz SJS, Vilela RD, dos Santos JM, Barbosa GVDS, Silva JAC (2016) Foliar applications of calcium reduce and delay sugarcane flowering. J BioEnergy Res 9:98–108
Eriksson S, Böhlenius H, Moritz T, Nilsson O (2006) GA4 is the active gibberellin in the regulation of LEAFY transcription and Arabidopsis floral initiation. J Plant Cell 18:2172–2181
Fang J, Chai Z, Yao W, Chen B, Zhang M (2021) Interactions between ScNAC23 and ScGAI regulate GA-mediated flowering and senescence in sugarcane. J Plant Sci 304:110806
Farinas B, Mas P (2011) Functional implication of the MYB transcription factor RVE8/LCL5 in the circadian control of histone acetylation. J Plant J 66:318–329
Farré EM, Harmer SL, Harmon FG, Yanovsky MJ, Kay SA (2005) Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis circadian clock. J Curr Biol 15:47–54
Fornara F, Panigrahi KC, Gissot L, Sauerbrunn N, Rühl M, Jarillo JA, Coupland G (2009) Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. J Dev Cell 17:75–86
Gajdanowicz P, Michard E, Sandmann M, Rocha M, Corrêa LGG, Ramírez- Aguilar SJ, Gomez-porras JL, Gonzalez W, Thibaud J, Van Dongen JT, Dreyer I (2011) Potassium (K+) gradients serve as a mobile energy source in plant vascular tissues. Proc Natl Acad Sci 108:864–869
Galvão VC, Collani S, Horrer D, Schmid M (2015) Gibberellic acid signaling is required for ambient temperature-mediated induction of flowering in Arabidopsis thaliana. Plant J 84:949–962
Gandikota M, Birkenbihl RP, Höhmann S, Cardon GH, Saedler H, Huijser P (2007) The miRNA156/157 recognition element in the 3′ UTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. J Plant J 49:683–693
Giaimo BD, Ferrante F, Herchenröther A, Hake SB, Borggrefe T (2019) The histone variant H2A. Z in gene regulation. J Epigenet Chrom 12:1–22
Glassop D, Rae AL, Bonnett GD (2014) Sugarcane flowering genes and pathways in relation to vegetative regression. J Sugar Tech 16:235–240
Gosnell JM (1973) Some factors affecting flowering in sugarcane. Proc South Afr Sugar Technol Assoc 47:144–147
Gou J, Tang C, Chen N, Wang H, Debnath S, Sun L, Flanagan A, Tang Y, Jiang Q, Allen RD, Wang ZY (2019) SPL 7 and SPL 8 represent a novel flowering regulation mechanism in switchgrass. J New Phytologist 222:1610–1623
Gururaja Rao PN, Shanmugavadivu R, Vasantha S (2012) RNA Content in relation to flowering in sugarcane varieties. J Sugar Tech 14:83–85
Hale AL, White PM, Webber CL III, Todd JR (2017) Effect of growing media and fertilization on sugarcane flowering under artificial photoperiod. J Plos One 12:e0181639
Hanzawa Y, Money T, Bradley D (2005) A single amino acid converts a repressor to an activator of flowering. Proc Natl Acad Sci 102:7748–7753
Harig L, Beinecke FA, Oltmanns J, Muth J, Müller O, Rüping B, Twyman RM, Fischer R, Prufer D, Noll GA (2012) Proteins from the FLOWERING LOCUS T-like subclade of the PEBP family act antagonistically to regulate floral initiation in tobacco. Plant J 72:908–921
Harmer SL (2009) The circadian system in higher plants. J Ann Rev Plant Biol 60:357–377
Hauvermale AL, Ariizumi T, Steber CM (2012) Gibberellin signaling: a theme and variations on DELLA repression. J Plant Physiol 160:83–92
Hayama R, Coupland G (2003) Shedding light on the circadian clock and the photoperiodic control of flowering. J Curr Opin Plant Biol 6:13–19
Helfer A, Nusinow DA, Chow BY, Gehrke AR, Bulyk ML, Kay SA (2011) LUX ARRHYTHMO encodes a nighttime repressor of circadian gene expression in the Arabidopsis core clock. J Curr Biol 21:126–133
Hepworth SR, Valverde F, Ravenscroft D, Mouradov A, Coupland G (2002) Antagonistic regulation of flowering-time gene SOC1 by CONSTANS and FLC via separate promoter motifs. EMBO J 21:4327–4337
Hill CB, Li C (2016) Genetic architecture of flowering phenology in cereals and opportunities for crop improvement. J Front Plant Sci 7:1906
Hyun Y, Richter R, Coupland G (2017) Competence to flower: age-controlled sensitivity to environmental cues. J Plant Physiol 173:36–46
Imaizumi T (2010) Arabidopsis circadian clock and photoperiodism: time to think about location. J Curr Opin Plant Biol 13:83–89
Imaizumi T, Tran HG, Swartz TE, Briggs WR, Kay SA (2003) FKF1 is essential for photoperiodic-specific light signalling in Arabidopsis. J Nat 426:302–306
Imaizumi T, Schultz TF, Harmon FG, Ho LA, Kay SA (2005) FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. J Sci 309:293–297
James NI, Smith GA (1969) Effect of photoperiod and light intensity on flowering in sugarcane. J Crop Sci 9:794–796
Jang S, Marchal V, Panigrahi KC, Wenkel S, Soppe W, Deng XW, Valverde F, Coupland G (2008) Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. J EMBO J 27:1277–1288
Jin S, Ahn JH (2021) Regulation of flowering time by ambient temperature: repressing the repressors and activating the activators. J New Phytol 230:938–942
Jones-Rhoades MW, Bartel DP, Bartel B (2006) MicroRNAs and their regulatory roles in plants. J Ann Rev Plant Biol 57:19–53
Julien MHR (1971) The photoperiodic control of flowering in Saccharum. Proc Int Soc Sug Cane Technol 14:223–233
Jung JH, Seo PJ, Kang SK, Park CM (2011) miR172 signals are incorporated into the miR156 signaling pathway at the SPL3/4/5 genes in Arabidopsis developmental transitions. Plant Mol Biol 76:35–45
Jung JH, Seo YH, Seo PJ, Reyes JL, Yun J, Chua NH, Park CM (2007) The GIGANTEA-regulated microRNA172 mediates photoperiodic flowering independent of CONSTANS in Arabidopsis. J Plant Cell 19:2736–2748
Jung JH, Ju Y, Seo PJ, Lee JH, Park CM (2012a) The SOC1-SPL module integrates photoperiod and gibberellic acid signals to control flowering time in Arabidopsis. J Plant J 69:577–588
Jung JH, Ju Y, Seo PJ, Lee JH, Park CM (2012b) The SOC1-SPL module integrates photoperiod and gibberellic acid signals to control flowering time in Arabidopsis. Plant J 69:577–588
Karlgren A, Gyllenstrand N, Källman T, Sundström JF, Moore D, Lascoux M, Lagercrantz U (2011) Evolution of the PEBP gene family in plants: functional diversification in seed plant evolution. J Plant Physiol 156:1967–1977
Kim WY, Fujiwara S, Suh SS, Kim J, Kim Y, Han L, David K, Putterill J, Nam HG, Somers DE (2007) ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. J Nat 449:356–360
Kim JJ, Lee JH, Kim W, Jung HS, Huijser P, Ahn JH (2012) The microRNA156-SQUAMOSA PROMOTER BINDING PROTEIN-LIKE3 module regulates ambient temperature-responsive flowering via FLOWERING LOCUS T in Arabidopsis. J Plant Physiol 159:461–478
Kumar SV, Wigge PA (2010) H2A. Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. J Cell 140:136–147
Kumar SV, Lucyshyn D, Jaeger KE, Alós E, Alvey E, Harberd NP, Wigge PA (2012) Transcription factor PIF4 controls the thermosensory activation of flowering. J Nat 484:242–245
Lariguet P, Dunand C (2005) Plant photoreceptors: phylogenetic overview. J Mol Evol 61:559–569
Laubinger S, Marchal V, Gentilhomme J, Wenkel S, Adrian J, Jang S, Kulajta C, Braun H, Coupland G, Hoecker U (2006) Arabidopsis SPA proteins regulate photoperiodic flowering and interact with the floral inducer CONSTANS to regulate its stability. J Dev 133:3213–3222
Lazaro A, Valverde F, Piñeiro M, Jarillo JA (2012) The Arabidopsis E3 ubiquitin ligase HOS1 negatively regulates CONSTANS abundance in the photoperiodic control of flowering. J Plant Cell 24:982–999
Lee J, Lee I (2010) Regulation and function of SOC1, a flowering pathway integrator. J Exp Bot 61(9):2247–2254
Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH (2007) Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. J Genes Dev 21:397–402
Legnaioli T, Cuevas J, Mas P (2009) TOC1 functions as a molecular switch connecting the circadian clock with plant responses to drought. EMBO J 28(23):3745–3757
Li QH, Yang HQ (2007) Cryptochrome signalling in plants. J Photochem Photobiol 83:94–101
Li D, Liu C, Shen L, Wu Y, Chen H, Robertson M, Helliwell CA, Ito T, Meyerowitz E, Yu H (2008) A repressor complex governs the integration of flowering signals in Arabidopsis. J Dev Cell 15:110–120
Linhares-Neto MV, Schumacher PV, Ribeiro THC, Cardon CH, Resende PM, Chalfun-Junior A (2021) ScFT6: a putative candidate for sugarcane floral inducer
Liu LJ, Zhang YC, Li QH, Sang Y, Mao J, Lian HL, Wang L, Yang HQ (2008) COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis. J Plant Cell 20:292–306
Long AC (1976) A large varietal difference in cane deterioration due to flowering. Pro South Afr Sugar Technol Assoc 50:78–81
Lu SX, Webb CJ, Knowles SM, Kim SH, Wang Z, Tobin EM (2012) CCA1 and ELF3 Interact in the control of hypocotyl length and flowering time in Arabidopsis. J Plant Physiol 158:1079–1088
Ma Z, Li W, Wang H, Yu D (2020) WRKY transcription factors WRKY12 and WRKY13 interact with SPL10 to modulate age-mediated flowering. J Integr Plant Biol 62:1659–1673
Más P, Kim WY, Somers DE, Kay SA (2003) Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana. J Nat 426:567–570
McClung CR (2006) Plant circadian rhythms. J Plant Cell 18:792–803
McClung CR (2011) The genetics of plant clocks. Adv Genet 74:105–139
McWatters HG, Devlin PF (2011) Timing in plants–a rhythmic arrangement. J FEBS Lett 585:1474–1484
Melloni MLG, Melloni MNG, Scarpari MS, Garcia JC, Landell MG, Pinto LR (2015) Flowering of sugarcane genotypes under different artificial photoperiod conditions. J Am J Plant Sci 6:456–463
Menshawi ZA (1978) Chemical constituents of the sugarcane apex associated with floral evocation. Proc Int Assoc Sugar Cane Technol 16:1885–1901
Miah MAS, Sarkar MAA (1981) Effect of flowering on the quality of sugarcane. J Bangl J Sugarcane 3:25–28
Midmore DJ (1980) Effects of photoperiod on flowering and fertility of sugarcane (Saccharum spp.). J Field Crops Res 3:65–81
Ming R, Del Monte TA, Hernandez E, Moore PH, Irvine JE, Paterson AH (2002) Comparative analysis of QTLs affecting plant height and flowering among closely-related diploid and polyploid genomes. Genome 45(5):794–803
Moon J, Suh SS, Lee H, Choi KR, Hong CB, Paek NC, Kim S, Lee I (2003) The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J 35(5):613–623
Moore PH, Berding N (2014) Flowering. In: Moor PH, Botha FC (eds) Sugarcane: Physiology, biochemistry, and functional biology, John Wiley & Sons, Inc. (Iowa), pp 379–410
Mukherjee SK (1957) Origin and distribution of Saccharum. J Bot Gazette 119:55–61
Mutasa-Göttgens E, Hedden P (2009) Gibberellin as a factor in floral regulatory networks. J Exp Bot 60:1979–1989
Nakamichi N, Kiba T, Henriques R, Mizuno T, Chua NH, Sakakibara H (2010) PSEUDO-RESPONSE REGULATORS 9, 7, and 5 are transcriptional repressors in the Arabidopsis circadian clock. J Plant Cell 22:594–605
Nayamuth R, Mangar M, Soopaya R (2003) Characterization of natural environments for sugarcane flowering ability. AMAS Food and Agricultural Research Council, Reduit, Mauritius 179–187
Nuss KJ, Berding N (1999) Planned recombination in sugarcane breeding: artificial initiation of flowering in sugarcane in sub-tropical and tropical conditions. Proc Int Soc Sug Cane Technol 23:504–508
Panje RR, Srinivasan K (1959) Studies in Saccharum spontaneum. The flowering behavior of latitudinally displaced populations. J Bot Gazette 120:193–202
Patil SB, Guddadamath SG, Khadi BM (2014) Genetic enhancement of sugarcane productivity combining non flowering feature. Sugar Tech. https://doi.org/10.1007/s12355-014-0338-x
Pin PA, Benlloch R, Bonnet D, Wremerth-Weich E, Kraft T, Gielen JJ, Nilsson O (2010) An antagonistic pair of FT homologs mediates the control of flowering time in sugar beet. J Sci 330:1397–1400
Price Policy for Sugarcane 2022–23 Sugar Season. Commission for Agricultural costs and Prices. Department of Agriculture and Farmers Welfare, Ministry of Agriculture and Farmers welfare, GOI, December 2021. https://cacp.dacnet.nic.in/Default.aspx#
Pose D, Verhage L, Ott F, Yant L, Mathieu J, Angenent GC, Immink RGH, Schmid M (2013) Temperature-dependent regulation of flowering by antagonistic FLM variants. J Nat 503:414–417
Premachandran MN, Prathima PT, Lekshmi M (2011) Sugarcane and polyploidy: a review. J Sugarcane Res 1:1–15
Rai KK (2022) Integrating speed breeding with artifcial intelligence for developing climate-smart crops. Mol Biol Rep. https://doi.org/10.1007/s11033-022-07769-4
Rao PS (1977) Effects of flowering on yield and quality of sugarcane. J Exp Agric 13:381–387
Rao PN, Naresh Kumar K (2003) Effect of flowering on juice quality and fibre content in sugarcane. J Sugar Tech 5:185–187
Rawat R, Takahashi N, Hsu PY, Jones MA, Schwartz J, Salemi MR, Phinney BS, Harmer SL (2011) REVEILLE8 and PSEUDO-REPONSE REGULATOR5 form a negative feedback loop within the Arabidopsis circadian clock. J PLoS Genet 7:e1001350
Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, Bartel DP (2002) Prediction of plant microRNA targets. J Cell 110:513–520
Samach A, Wigge PA (2005) Ambient temperature perception in plants. J Curr Opin Plant Biol 8:483–486
Sawa M, Nusinow DA, Kay SA, Imaizumi T (2007) FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. J Sci 318:261–265
Searle I, He Y, Turck F, Vincent C, Fornara F, Kröber S, Amasino RA, Coupland G (2006) The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev 20(7):898–912
Serivichyaswat P, Ryu HS, Kim W, Kim S, Chung KS, Kim JJ, Ahn JH (2015) Expression of the floral repressor miRNA156 is positively regulated by the AGAMOUS-like proteins AGL15 and AGL18. J Mol Cells 38:259
Singh P, Singh P, Singh J (2019) Effect of arrowing/flowering on juice quality of sugarcane. J Indian J Sugarcane Technol 34:82–84
Singh S, Naidu MK, Tyagi DN (1986) Effect of auxin, anti-auxin and metabolic inhibitor on the flowering of early and late flowering varieties of sugar cane. Congress of the International Society of Sugar Cane Technologists
Somers DE, Devlin PF, Kay SA (1998) Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. J Sci 282:1488–1490
Song YH, Shim JS, Kinmonth-Schultz HA, Imaizumi T (2015) Photoperiodic flowering: time measurement mechanisms in leaves. J Ann Rev Plant Biol 66:441–464
Srikanth A, Schmid M (2011) Regulation of flowering time: all roads lead to Rome. J Cell Mol Life Sci 68:2013–2037
Srivastava D, Shamim M, Kumar M, Mishra A, Maurya R, Sharma D, Pandey P, Singh KN (2019) Role of circadian rhythm in plant system: an update from development to stress response. J Environ Exp Botany 162:256–271
Staiger D, Shin J, Johansson M, Davis SJ (2013) The circadian clock goes genomic. Genome Biol 14:208–9801
Strasser B, Alvarez MJ, Califano A, Cerdán PD (2009) A complementary role for ELF3 and TFL1 in the regulation of flowering time by ambient temperature. Plant J 58:629–640
Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G (2001) CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. J Nat 410:1116–1120
Sun G (2012) MicroRNAs and their diverse functions in plants. J Plant Mol Biol 80:17–36
Teotia S, Tang G (2015) To bloom or not to bloom: role of microRNAs in plant flowering. J Mol Plant 8:359–377
Thines B, Harmon FG (2010) Ambient temperature response establishes ELF3 as a required component of the core Arabidopsis circadian clock. Proc Natl Acad Sci 107:3257–3262
Thines BC, Youn Y, Duarte MI, Harmon FG (2014) The time of day effects of warm temperature on flowering time involve PIF4 and PIF5. J Exp Bot 65:1141–1151
Thulijaram R (1964) Flowering in sugarcane is not harmful if crop is harvested within three months. J Shakti Sugar News 1:5–6
Valverde F (2011) CONSTANS and the evolutionary origin of photoperiodic timing of flowering. J Exp Bot 62:2453–2463
Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G (2004) Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. J Sci 303:1003–1006
Venail J, da Silva SPH, Manechini JR, Alves LC, Scarpari M, Falcão T, Romanel E, Brito M, Vicentini R, Pinto L, Jackson SD (2022) Analysis of the PEBP gene family and identification of a novel FLOWERING LOCUS T orthologue in sugarcane. J Exp Bot 73:2035–2049
Wang JW (2014) Regulation of flowering time by the miR156-mediated age pathway. J Exp Botany 65:4723–4730
Wang JW, Czech B, Weigel D (2009) miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. J Cell 138:738–749
Webb AAR (2003) The physiology of circadian rhythms in plants. J New Phytologist 160:281–303
Wellmer F, Riechmann JL (2010) Gene networks controlling the initiation of flower development. J Trends Genet 26:519–527
Wickland DP, Hanzawa Y (2015) The FLOWERING LOCUS T/TERMINAL FLOWER 1 gene family: functional evolution and molecular mechanisms. J Mol Plant 8:983–997
Wigge PA (2013) Ambient temperature signalling in plants. J Curr Opin Plant Biol 16:661–666
Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D (2005) Integration of spatial and temporal information during floral induction in Arabidopsis. J Sci 309:1056–1059
Wolabu TW, Zhang F, Niu L, Kalve S, Bhatnagar-Mathur P, Muszynski MG, Tadege M (2016) Three FLOWERING LOCUS T-like genes function as potential florigens and mediate photoperiod response in sorghum. J New Phytologist 210:946–959
Wu G, Poethig RS (2006) Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. J Dev 133:3539–3547
Wu G, Park MY, Conway SR, Wang JW, Weigel D, Poethig RS (2009) The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. J Cell 138:750–759
Xie Q, Wang P, Liu X, Yuan L, Wang L, Zhang C, Li Y, Xing H, Zhi L, Yue Z, Zhao C, McClung CR, Xu X (2014) LNK1 and LNK2 are transcriptional coactivators in the Arabidopsis circadian oscillator. J Plant Cell 26:2843–2857
Xu F, Li T, Xu PB, Li L, Du SS, Lian HL, Yang HQ (2016) DELLA proteins physically interact with CONSTANS to regulate flowering under long days in Arabidopsis. FEBS Lett 590:541–549
Yamaguchi A, Abe M (2012) Regulation of reproductive development by non-coding RNA in Arabidopsis: to flower or not to flower. J Plant Res 125:693–704
Yamaguchi A, Kobayashi Y, Goto K, Abe M, Araki T (2005) TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. J Plant Cell Physiol 46:1175–1189
Yamaguchi A, Wu MF, Yang L, Wu G, Poethig RS, Wagner D (2009) The microRNA-regulated SBP-Box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. J Dev Cell 17:268–278
Yoo SY, Kardailsky I, Lee JS, Weigel D, Ahn JH (2004) Acceleration of flowering by overexpression of MFT. J Mol Cell 17:95–101
Yuan C, Li H, Qin C, Zhang X, Chen Q, Zhang P, Xu X, He M, Zhang X, Tor M, Xue D, Wang H, Jackson S, He Y, Liu Y, Shi N, Hong Y (2020) Foxtail mosaic virus-induced flowering assays in monocot crops. J Exp Bot 71(10):3012–3023
Zagotta MT, Hicks KA, Jacobs CI, Young JC, Hangarter RP, Meeks-Wagner DR (1996) The Arabidopsis ELF3 gene regulates vegetative photomorphogenesis and the photoperiodic induction of flowering. Plant J 10:691–702
Zhang J, Nagai C, Yu Q, Pan YB, Ayala-Silva T, Schnell RJ, Comstock JC, Arumuganathan AK, Ming R (2012) Genome size variation in three Saccharum species. J Euphytica 185:511–519
Zhu QH, Helliwell CA (2011) Regulation of flowering time and floral patterning by miR172. J Exp Bot 62:487–495
Zuo Z, Liu H, Liu B, Liu X, Lin C (2011) Blue light-dependent interaction of CRY2 with SPA1 regulates COP1 activity and floral initiation in Arabidopsis. J Curr Biol 21:841–847
Author information
Authors and Affiliations
Contributions
PG, PKM conceived the idea of the review and surveyed the literature; PG, PKM, SKV drafted the manuscript equally; PKM, SKV scrutinized and corrected the manuscript to its final version. All the authors read and approved the final version of the manuscript prior to its submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pavani, G., Malhotra, P.K. & Verma, S.K. Flowering in sugarcane-insights from the grasses. 3 Biotech 13, 154 (2023). https://doi.org/10.1007/s13205-023-03573-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03573-4