Abstract
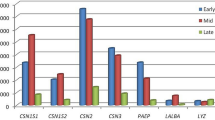
The molecular physiology of milk production of two important dairy species; Sahiwal cows (Bos indicus) and Murrah buffaloes (Bubalus bubalis) are not fully understood due to constraints in obtaining mammary tissue samples because of sacred and ethical reasons. The present study suggests the use of milk-derived mammary epithelial cells (MECs) as a non-invasive method to understand molecular aspects of lactation biology in dairy animals. A total of 76 MECs were collected from five different lactation periods viz. colostrum (0–2), early (5–20), peak (30–50), mid (90–140) and late lactation (> 215 days) stages from Sahiwal cows and Murrah buffaloes to study the transcription kinetics of milk protein, fat synthesis, and their regulatory genes. Significant changes were observed in milk composition of both dairy species with lactation stages. High mRNA abundance of all milk protein and fat synthesis genes was observed in MECs of Murrah buffaloes as compared to Sahiwal cows. The mRNA abundance of caseins (CSN1S1, CSN1S2, CSN2, and CSN3) and whey protein (LALBA, LF) were higher in early lactation stage. Similarly, the expression of milk fat synthesis genes (SCD, BTN1A1, ACACA, GPAM, FAPB3, FASN) was also high in early lactation stage. The relative abundance of 4 regulatory genes (JAK2, STAT5, SREBF1 and EIF4BP41) remained high during early lactation indicating their regulatory roles in lactogenesis process. Overall, results suggested a significant effect of lactation stages on milk composition and transcription abundance of milk protein and fat synthesis genes. The present study establishes the fact that milk-derived MECs could be utilized as a valuable source to understand mammary gland functioning of native cows and buffaloes.




Similar content being viewed by others
References
Al-Saiady MY (2006) Effect of restricted feeding, breed and diet on sheep milk yield. J Appl Anim Res 30:85–88
Bauman DE, Griinari JM (2003) Nutritional regulation of milk fat synthesis. Ann Rev Nut 23:203–227
Bevilacqua C, Helbling JC, Miranda G, Martin P (2006) Translational efficiency of casein transcripts in the mammary tissue of lactating ruminants. Reprod Nut Dev 46:567–578
Bionaz M, Loor JJ (2008) Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genom 9:366
Bionaz M, Loor JJ (2011) Gene networks driving bovine mammary protein synthesis during the lactation cycle. Bioinf Biol Insights 5:83–98
Boutinaud M, Guinard-Flament J (2004) The number and activity of mammary epithelial cells, determining factors for milk production. Rep Nut Dev 44:499–508
Boutinaud M, Chedly MB, Delamaire E, Guinard-Flament J (2008) Milking and feed restriction regulate transcripts of mammary epithelial cells purified from milk. J Dairy Sci 91:988–998
Capuco AV, Ellis SE, Hale SA, Long E, Erdman RA, Zhao X, Paape MJ (2003) Lactation persistency: Insights from mammary cell proliferation studies. J Anim Sci 81:18–31
Chilliard Y, Ferlay A, Mansbridge RM, Doreau M (2000) Ruminant milk fat plasticity: nutritional control of saturated, polyunsaturated, trans and conjugated fatty acids. In Annals Zootech 49:181–205
Colitti M, Pulina G (2010) Expression profile of caseins, estrogen and prolactin receptors in mammary. Ital J Anim Sci 9:e55
Dhiman TR, Zaman S, Olson KC, Bingham HR, Ure AL, Pariza MW (2005) Influence of feeding soybean oil on conjugated linoleic acid content in beef. J Agri Food Chem 53:684–689
El-Tarabany MS, El-Bayoumi KM (2015) Reproductive performance of backcross Holstein × Brown Swiss and their Holstein contemporaries under subtropical environmental conditions. Theriogenology 83:444–448
German JB, Dillard CJ (2006) Composition, structure and absorption of milk lipids: a source of energy, fat-soluble nutrients and bioactive molecules. Cri Rev Food Sci Nut 46:57–92
Heuer C, Schukken YH, Dobbelaar P (1999) Postpartum body condition score and results from the first test day milk as predictors of disease, fertility, yield, and culling in commercial dairy herds. J Dairy Sci 82:295–304
Holt C, Carver JA, Ecroyd H, Thorn DC (2013) Invited review: Caseins and the casein micelle: Their biological functions, structures, and behaviour in foods. J Dairy Sci 96:6127–6146
Idamokoro EM, Muchenje V, Masika PJ (2017) Yield and milk composition at different stages of lactation from a small herd of nguni, boer, and non-descript goats raised in an extensive production system. Sustainability 9:1000
Jatav P, Sodhi M, Sharma A, Mann S, Kishore A, Shandilya UK et al (2016) Identification of internal control genes in milk-derived mammary epithelial cells during lactation cycle of Indian zebu cow. Anim Sci J 87:344–353
Javed R, Gautam SK, Vijh RK, Tantia MS (2011) Characterization of PRLR and PPARGC1A genes in buffalo (Bubalus bubalis). Genet Mol Biol 34:592–594
Kadegowda AKG, Bionaz M, Piperova LS, Erdman RA, Loor JJ (2009) Peroxisome proliferator-activated receptor-γ activation and long-chain fatty acids alter lipogenic gene networks in bovine mammary epithelial cells to various extents. J Dairy Sci 92:4276–4289
Kay JK, Weber WJ, Moore CE, Bauman DE, Hansen LB, Chester-Jones H, Crooker BA, Baumgard LH (2005) Effects of week of lactation and genetic selection for milk yield on milk fatty acid composition in Holstein cows. J Dairy Sci 88:3886–3893
Kishore A, Sodhi M, Mukesh M, Mishra BP, Sobti RC (2013) Sequence analysis and identification of new variations in the 5′-flanking region of αS2-casein gene in Indian zebu cattle. Mol Biol Rep 40:4473–4481
Lemay DG, Neville MC, Rudolph MC, Pollard KS, German JB (2007) Gene regulatory networks in lactation: identification of global principles using bioinformatics. BMC Sys Biol 1:56
Liu X, Gallego MI, Smith GH, Robinson GW, Hennighausen L (1998) Functional rescue of Stat5a-null mammary tissue through the activation of compensating signals including Stat5b. Cell Growth Differ 9:795–804
McFadden JW, Corl BA (2010) Activation of liver X receptor (LXR) enhances de novo fatty acid synthesis in bovine mammary epithelial cells. J Dairy Sci 93:4651–4658
Mech A, Dhali A, Prakash B, Rajkhowa C (2008) Variation in milk yield and milk composition during the entire lactation period in Mithun cows (Bos frontalis). Livest Res Rural Dev 20:5
Menzies KK, Lee HJ, Lefèvre C, Ormandy CJ, Macmillan KL, Nicholas KR (2010) Insulin, a key regulator of hormone responsive milk protein synthesis during lactogenesis in murine mammary explants. Funct Integ Genomic 10:87–95
Nanda AS, Nakao T (2003) Role of buffalo in the socioeconomic development of rural Asia: Current status and future prospectus. Anim Sci J 74:443–455
Ntambi JM, Miyazaki M (2003) Recent insights into stearoyl-CoA desaturase-1. Curr Opin Lipidol 14:255–261
Oravcová M, Margetín M, Peskovicova D, Dano J, Milerski M, Hetényi L, Polák P (2007) Factors affecting ewe’s milk fat and protein content and relationships between milk yield and milk components. Cz J Anim Sci 52:189
Paten AM, Duncan EJ, Pain SJ, Peterson SW, Kenyon PR, Blair HT, Dearden PK (2015) Functional development of the adult ovine mammary gland—insights from gene expression profiling. BMC Genom 16:748
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acid Res 29:e45
Piantoni P, Wang P, Drackley JK, Hurley WL, Loor JJ (2010) Expression of metabolic, tissue remodeling, oxidative stress, and inflammatory pathways in mammary tissue during involution in lactating dairy cows. Bioinfo Biol Insights 4:85–97
Riley LG, Williamson P, Wynn PC, Sheehy PA (2008) Lactoferrin decreases primary bovine mammary epithelial cell viability and casein expression. J Dairy Res 75:135–141
Rudolph MC, Monks J, Burns V, Phistry M, Marians R, Foote MR, Bauman DE, Anderson SM, Neville MC (2010) Sterol regulatory element binding protein and dietary lipid regulation of fatty acid synthesis in the mammary epithelium. Am J Physiol Endocrin Metabol 299:E918–E927
Schanbacher FL, Talhouk RS, Murray FA (1997) Biology and origin of bioactive peptides in milk. Livest Prod Sci 50:105–123
Sharma A, Aggarwal J, Sodhi M, Kishore A et al (2014) Stage specific expression of ATP-binding cassette and solute carrier superfamily of transporter genes in mammary Gland of riverine buffalo (Bubalus bubalis). Anim Biotech 25:200–209
Sigl T, Meyer HHD, Wiedemann S (2012) Gene expression of six major milk proteins in primary bovine mammary epithelial cells isolated from milk during the first twenty weeks of lactation. Czech J Anim Sci 57:469–480
Silva SV, Malcata FX (2005) Caseins as source of bioactive peptides. Int Dairy J 15:1–15
Singh B, Chauhan MS, Singla SK, Gautam SK et al (2009) Reproductive biotechniques in buffaloes (Bubalus bubalis): status, prospects and challenges. Reprod Fertility Dev 21:499–510
Siqueiros-Cendón T, Arévalo-Gallegos S, Iglesias-Figueroa BF, García-Montoya IA, Salazar-Martínez J, Rascón-Cruz Q (2014) Immunomodulatory effects of lactoferrin. Acta Pharmacol Sin 35:557–566
Slots T, Butler G, Leifert C, Kristensen T, Skibsted LH, Nielsen JH (2009) Potentials to differentiate milk composition by different feeding strategies. J Dairy Sci 92:2057–2066
Sodhi M, Mukesh M, Prakash B, Mishra BP et al (2007) MspI allelic pattern of bovine growth hormone gene in Indian Zebu cattle (Bos indicus) breeds. Biochem Genet 45:145–153
Stoop WM, Bovenhuis H, Heck JML, Van Arendonk JAM (2009) Effect of lactation stage and energy status on milk fat composition of Holstein-Friesian cows. J Dairy Sci 92:1469–1478
Strzałkowska N, Jóźwik A, Bagnicka E, Krzyżewski J, Horbańczuk K, Pyzel B, Horbańczuk JO (2009) Chemical composition, physical traits and fatty acid profile of goat milk as related to the stage of lactation. Anim Sci Papers Reports 27:311–320
Suárez-Vega A, Gutiérrez-Gil B, Klopp C, Robert-Granie C, Tosser-Klopp G, Arranz JJ (2015) Characterization and comparative analysis of the milk transcriptome in two dairy sheep breeds using RNA sequencing. Sci Rep 5:18399
Szewczuk M (2015) Association of a genetic marker at the bovine Janus kinase 2 loci (JAK2/RsaI) with milk production traits of four cattle breeds. J Dairy Res 82:287–292
Toerien CA, Cant JP (2007) Abundance and phosphorylation state of translation initiation factors in mammary glands of lactating and non-lactating dairy cows. J Dairy Sci 90:2726–2734
Wheeler TT, Broadhurst MK, Sadowski HB, Farr VC, Prosser CG (2001) Stat5 phosphorylation status and DNA-binding activity in the bovine and murine mammary glands. Mol Cell Endocrin 176:39–48
Wickramasinghe S, Rincon G, Islas-Trejo A, Medrano JF (2012) Transcriptional profiling of bovine milk using RNA sequencing. BMC Genom 13:45
Yang J, Jiang J, Liu X, Wang H, Guo G, Zhang Q, Jiang L (2016) Differential expression of genes in milk of dairy cattle during lactation. Anim Genet 47:74–180
Zicarelli L (2004) Buffalo milk: Its properties, dairy yield, and Mozzarella production. Vet Res Comm 28:127–135
Acknowledgements
The work was supported by Indian Council of Agriculture Research, New Delhi under the National Agriculture Innovation Project. The authors duly acknowledge Director, National Bureau of Animal Genetic Resources for providing the research facilities to carry out this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Sharma, A., Shandilya, U.K., Sodhi, M. et al. Milk-derived mammary epithelial cells as non-invasive source to define stage-specific abundance of milk protein and fat synthesis transcripts in native Sahiwal cows and Murrah buffaloes. 3 Biotech 9, 106 (2019). https://doi.org/10.1007/s13205-019-1642-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-019-1642-7