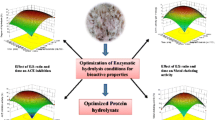
Abstract
This study examines the extraction and hydrolysis of proteins using single factor and Box–Behnken Design (BBD). From single factor tests, optimised extraction parameters were 1% alkali concentration, 40 °C temperature, 60 min time, and 1:20 solid to alkali ratio. Under these conditions; 924.31 mg/g of total protein was obtained from Limonia acidissima (L acidissima). The maximum degree of hydrolysis was 39.82% at pH 2, enzyme to substrate ratio 2.5% (w/w), and hydrolysis time was 42.41 min using BBD design. L acidissima seed protein hydrolysate showed 32.94% DPPH and 88.18% of ABTS activity at concentration of 100 µg/ml and 1 mg/ml, respectively. Reducing power of 0.16 and metal chelating activity of 87.39% was obtained from 5 mg/ml protein hydrolysates. This implied that L acidissima seed protein hydrolysate could be utilised in protein rich product or as protein supplements.




Similar content being viewed by others
References
Adler-Nissen J (1986) Enzymic hydrolysis of food proteins. Elsevier Applied Science, London
Ahamed SM, Swamy SK, Jayaverra KN, Rao JV, Kumar S (2008) Anti inflammatory, antipyretic and analgesic activity of methanolic extract of Feronia limonia. Pharmacol 3:852–857
Arnao MB (2000) Some methodological problems in the determination of antioxidant activity using chromogen radicals: a practical case. Trends Food Sci Technol 11:419–421
Atkinson AC, Donev AN (1992) Optimum experimental designs. Clarendon Press, Oxford
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Box GEP, Draper NR (1987) Empirical model-building and response surfaces. Wiley, New York, p 249
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen HM, Muramoto K, Yamauchi F, Nokihara K (1996) Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. J Agricul Food Chem 44(9):2619–2623
Decker EA, Welch B (1990) Role of ferritin as a lipid oxidation catalyst in muscle food. J Agricul Food Chem 38:674–677
Deng J, Sun T, Cao W, Fan D, Cheng N, Wang B, Gao H, Yang H (2014) Extraction optimization and functional properties of proteins from kiwi fruit (Actinidia chinensis Planch.) Seeds. Int J Food Prop 17(7):1612–1625
Elias RJ, Kellerby SS, Decker EA (2008) Antioxidant activity of proteins and peptides. Crit Rev Food Sci Nutr 48(5):430–441
Hochstrasser DF, Harrington MG, Hochstrasser AC, Miller MJ, Merril CR (1988) Methods for increasing the resolution of two-dimensional protein electrophoresis. Anal Biochem 173(2):424–435
Hoyle NT, Merritt JOHN (1994) Quality of fish protein hydrolysates from herring (Clupea harengus). J Food Sci 59(1):76–79
Kanbargi KD, Sonawane SK, Arya SS (2016) Functional and antioxidant activity of Ziziphus jujube seed protein hydrolysates. Food Measure 10:226–235
Kong BH, Xiong YL (2006) Antioxidant activity of zein hydrolysate in liposome system and the possible mode of action. J Agricul Food Chem 54:6059–6068
Kong XZ, Zhou HM, Hua YF (2008) Preparation and antioxidant activity of wheat gluten hydrolysates (WGHs) using ultrafiltration membranes. J Sci Food Agricul 88(5):920–926
Loomis WD, Battaile J (1966) Plant phenolic compounds and the isolation of plant enzymes. Phytochem 5(3):423–438
Lowry OH, Rosebrough NJ, Farr AL, Randal RJ (1951) Protein analysis with Folin-phenol reagent. J Biol Chem 193:265–275
Lv C, Jia X, Li M, Yang J, Zhao G (2011) Optimization of extraction process of crude protein from grape seeds by RSM. Food Sci Technol Int 17(5):437–445
Matsui T, Yukiyoshi A, Doi S, Sugimoto H, Yamada H, Matsumoto K (2002) Gastrointestinal enzyme production of bioactive peptides from royal jelly protein and their antihypertensive ability in SHR. J Nut Biochem 13:80–86
Morton JF (1987) Wood-Apple. In: Fruits of warm climates, Flare Books, Miami, Florida, p 190–191
Patel DK, Kumar R, Laloo D, Hemalatha S (2012) Diabetes mellitus: an overview on its pharmacological aspects and reported medicinal plants having ant diabetic activity. Asian Pac J Tropical Biomed 2(5):411–420
Prior RL, Wu X, Schaich K (2005) Standardized method for the determination of antioxidant capacities and phenolics in foods and dietary supplements. J Agricul Food Chem 53:4290–4302
Rahman MM, Gray AI (2002) Antimicrobial constituents from the stem bark of Feronia limonia. Phytochem 59(1):73–77
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Ryan JT, Ross RP, Bolton D, Fitzgerald GF, Stanton C (2011) Bioactive peptides from muscle sources. Meat Fish 3:756–791
Sahreen S, Khan M, Khan R (2010) Evaluation of antioxidant activities of various solvent extracts of Carissa opaca fruits. Food Chem 122:1205–1211
Saiga A, Tanabe S, Nishimura T (2003) Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J Agricul Food Chem 51:3661–3667
Saima Y, Das AK, Sarkar KK, Sen AK, Sur P (2000) An antitumor pectic polysaccharide from Feronia limonia. Int J Biol Macromolec 27(5):333–335
Sarmadi BH, Ismail A (2010) Antioxidative peptides from food proteins. Peptides 31:1949–1956
Sonawane S, Arya SS (2013) Antioxidant activity of jambhul, L acidissima, ambadi and ambat chukka: an indigenous lesser known fruits and vegetables of India. Adv Food Sci Technol 5(3):270–275
Sonawane SK, Arya SS (2015) Effect of drying and storage on bioactive components of jambhul and wood apple. J Food Sci Technol 52:2833–2841
Sonawane SK, Bagul MB, LeBlanc JG, Arya SS (2016) Nutritional, functional, thermal and structural characteristics of Citrullus lanatus and Limonia acidissima seed flours. Food Measu 10(1):72–79
Suetsuna K, Nakano T (2000) Identification of an antihypertensive peptide from peptic digest of wakame. J Nut Biochem 11:450–454
Wani AA, Sogi DS, Grover L, Saxena DC (2006) Effect of temperature, alkali concentration, mixing time and meal/solvent ratio on the extraction of watermelon seed proteins—a response surface approach. Biosyst Eng 94:67–73
Xie Z, Huang J, Xu X, Jin Z (2008) Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem 111:370–376
Zhang M, Gao J, Yang H (2009) Functional properties of 7 s globulin extracted from cowpea vicilins. Cereal Chem 3:261–266
Zhu LJ, Chen J, Tang XY, Xiong YL (2008) Reducing, radical scavenging, and chelation properties of in vitro digests of alcalase treated zein hydrolysate. J Agricul Food Chem 56:2714–2721
Acknowledgements
The research is funded by University Grant Commission (UGC), Government of India. We are also thankful to Advance Enzyme, Mumbai, India for providing enzymes as gift samples during this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
We do not have any conflicts of interest.
Rights and permissions
About this article
Cite this article
Sonawane, S.K., Arya, S.S. Bioactive L acidissima protein hydrolysates using Box–Behnken design. 3 Biotech 7, 218 (2017). https://doi.org/10.1007/s13205-017-0862-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0862-y