Abstract
To assess the genetic diversity among four canola cultivars (namely, Serw-3, Serw-4, Misser L-16 and Semu 249), random amplified polymorphic DNA (RAPD), simple sequence repeat polymorphism (SSR) and amplified fragment length polymorphism (AFLP) analyses were performed. The data indicated that all of the three molecular markers gave different levels of polymorphism. A total of 118, 31 and 338 markers that show 61, 67.7 and 81 % polymorphism percentages were resulted from the RAPD, SSR and AFLP analyses, respectively. Based on the data obtained the three markers can be used to differentiate between the four canola cultivars. The genotype-specific markers were determined, 18 out of the 72 polymorphic RAPD markers generated were found to be genotype-specific (25 %). The highest number of RAPD specific markers was scored for Semu 249 (15 markers), while Serw-4 scored two markers. On the other hand, Serw-3 scored one marker. The cultivar Semu 249 scored the highest number of unique AFLP markers, giving 57 unique markers, followed by Misser L-16 which was characterized by 40 unique AFLP markers, then Serw-3 giving 31 unique markers. While Serw-4 was characterized by the lowest number producing 14 unique positive markers. The dendrogram built on the basis of combined data from RAPD, SSR and AFLP analysis represents the genetic distances among the four canola cultivars. Understanding the genetic variability among the current canola cultivars opens up a possibility for developing a molecular genetic map that will lead to the application of marker-assisted selection tools in genetic improvement of canola.
Similar content being viewed by others
Introduction
Canola (Brassica napus L.) is considered as the most important source of vegetable oil and protein-rich meal worldwide. It was developed through conventional plant breeding from rapeseed. It ranks the third among the oil crops, following palm oil and soya oil and the fifth among economically important crops, following rice, wheat, maize and cotton (Sovero 1993; Stoutjesdijk et al. 2000). There are increased domestic and export market opportunities for canola oil that can be realized through the development of high-oleic acid canola to replace saturated palm oil in food service applications (Spector 1999; Stoutjesdijk et al. 2000). In addition, high-oleic acid oils are more nutritionally beneficial because oleic acid had cholesterol-lowering properties, whereas saturated fatty acids tend to raise blood cholesterol levels (Stoutjesdijk et al. 2000).
Egypt recently experienced a solid decline in the total oilseed production on account of reduced cotton area, which overwhelmed increases in soybean output (Hassan and Sahfique 2010). This increased demand, and the need for crop diversification, will undoubtedly promote increased acreage of canola in Egypt. According to the Egyptian Ministry of Agriculture and Land Reclamation (2003–07), the seed oil content in the canola cultivar Serw-4 riches 42 %, while the cultivar Serw-3 have 40 % therefore these two cultivars seems to be promising for its high oil contents. In Egypt, there are agricultural opportunities to increase canola production by expanding into the new reclaimed regions.
Traditional breeding strategies that have attempted to utilize genetic variation arising from varietal germplasm, induced mutations and somaclonal variations of cell and tissue cultures have met with only limited success (Kebede et al. 2010). Therefore, the methods that evaluate and identify the genotypes more precisely during the growing season, especially at early stages, are preferred by plant breeders (Charcosst and Moreau 2004; Basunanda et al. 2007).
The analysis of genetic variation and relatedness in germplasm are of great value for genetic resources conservation and plant breeding programs to determine the best crosses between different genotypes. Over the years, the methods for assessing genetic diversity have ranged from classical strategies such as morphological analysis to biochemical and molecular techniques (Marijanovic et al. 2009).
In recent years, the identification of Brassica cultivars has depended on the application of different DNA markers. As DNA sequences are independent of environmental conditions, identification can be determined at any stage of plant growth (Ahmad et al. 2007; Younessi et al. 2011).
DNA markers reflect directly individual differences at the level of DNA molecules, and cover coding and non-coding regions of the genome (Dandelj et al. 2004). They are not affected by environment, developmental stage, certain tissue and organ, and have high-genomic frequency, high polymorphism and mostly a random genomic distribution (Charcosst et al. 2004; Zeng et al. 2004).
Several molecular techniques have been developed to assess genetic diversity and discriminate between genotypes in different crops. These include restriction fragment length polymorphism (RFLP) (Jaroslava et al. 2002), random amplified polymorphic DNA (RAPD) (Ahmad et al. 2007), amplified fragment length polymorphism (AFLP) (Vos et al. 1995) and microsatellites or simple sequence repeat polymorphism (SSR) (Halton et al. 2002).
The objectives of this investigation were to determine the genetic variability among four canola cultivars (namely Serw-3, Serw-4, Misser L-16 and Semu 249) at the molecular levels using RAPD, SSR and AFLP markers and to use the combined data to construct a phylogenetic tree. The genotype-specific markers were also determined.
Materials and methods
Plant materials
Four canola genotypes, namely Serw-3, Serw-4, Misser L-16 and Semu 249 were kindly provided by Field Crop Institute, Agricultural Research Center, Ministry of Agriculture, Egypt.
RAPD analysis
DNA extraction
Genomic DNA was isolated from young leaves of greenhouse grown plants using the CTAB method early described in Rogers and Bendich (1985). The quality and quantity of DNA were determined using agarose gel (0.8 %) electrophoresis and spectrophotometer (Table 1).
PCR analysis
PCR reactions were performed in a total volume of 20 μl containing 10 ng DNA, 200 μM dNTPs, 1 μM of 15 arbitrary 10-mer primers (Operon Technology, Inc., Alameda, CA, USA), 0.5 units of Red Hot Taq polymerase (AB gene House, UK) and 10 × Taq polymerase buffer (AB gene House, UK). For DNA amplification Biometra thermal cycler (2720) was programmed as follows: 94 ºC for 5 min followed by 35 cycles 94 ºC for 1 min, 35 ºC for 1 min, 72 °C for 1 min and 72 ºC for 7 min.
The amplification products were analyzed by electrophoresis in 1 % agarose in TAE buffer, stained by ethidium bromide and photographed under UV light. The sequence of the tested primers was as follows:
Simple sequence repeat polymorphism analysis PCR reaction mix includes the following: DNA, 10 ng/μl; 10 × buffer; 10 mM dNTPs; 50 mM MgCl2; 10 μM each of forward and reverse primers. The PCR profile starts with 95 °C for 5 min followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min extension at 72 °C for 2 min. A final extension 72 °C for 7 min was included. The PCR products were electrophoresed in a 2 % agarose gels (for SSRs) at 100 V. The gel was then stained in ethidium bromide for 30 min, and then observed on a UV trans-illuminator.
AFLP analysis
Amplified fragment length polymorphism was performed as described by Vos et al. (1995) using the GIBCO BRL system I (Cat. No. 10544) according to the manufacturer’s protocol.
Amplified fragment length polymorphism amplification products were separated in a vertical denaturing 6 % polyacrylamide gel in a Sequi-Gen Cell (Bio-Rad Laboratories Inc.) as described by Bassam et al. (1991).
Band scoring and cluster analysis
The RAPD, SSR and AFLP gel images were scanned using the Gel Doc 2000 Bio-Rad system and analyzed with Quantity One Software v 4.0.1 (Bio-Rad Laboratories, Hercules, Co. USA). The bands were sized and then binary coded by 1 or 0 for their presence or absence in each genotype. The systat ver. 7 computer program was used to calculate the pairwise differences matrix and plot the dendrogram among canola cultivars (Yang and Quiros 1993). Cluster analysis was based on similarity matrices obtained with the unweighed pair-group method (UPGMA) using the arithmetic average to estimate the dendrogram (Table 2).
Result and discussion
Recently, the identification of Brassica cultivars has depended on the application of different DNA markers. As DNA sequences are independent of environmental conditions, identification can be determined at any stage of plant growth (Ahmad et al. 2007). The RAPD markers are easier and quicker to use and preferred in applications where relationships between closely related breeding lines are of interest. This analysis detects nucleotide sequence polymorphisms in DNA using a single primer of arbitrary nucleotide sequence. DNA profiling with suitable RAPD primers could be used for identification and discrimination between of oilseed rape cultivars (Mailer et al. 1997; Ahmad et al. 2007). In the present work to study the genetic variability among four canola cultivars, RAPD analysis was performed. All the primer tested produced amplification products with variable band number. One hundred eighteen RAPD markers were obtained and out of them 72 were polymorphic (61 %) and can be considered as useful RAPD markers for the four canola cultivars used (Fig. 1; Table 3). The highest number of RAPD bands was recorded for primers OPE-M-13 (11 bands), followed by OPE-Q-14 (10 bands), while the lowest was scored for OPE-C-02 and OPE-G-14 (6 bands).
The genotype-specific RAPD markers for the different canola cultivars used are listed in Table 4; 18 out of the 72 polymorphic RAPD markers generated were found to be genotype-specific (25 %). The highest number of RAPD specific markers was scored for Semu 249 (15 markers), while Serw-4 scored two markers. On the other hand, Serw-3 scored one marker.
Microsatellite or SSR is another PCR-based marker which is preferred by many geneticists and plant breeders because of higher repeatability, co-dominant nature, specificity and having multiple alleles (Cheng et al. 2009). Cruz et al. (2007) employed the SSR markers to characterize the flowering time of spring and winter type B. napus L. germplasm. Qu et al. (2012) studied the genetic diversity and performed relationship analysis among the B. napus germplasm using SSR markers.
In the present study, seven SSR primer pairs flanking dinucleotide SSR (GA or AG) were used to investigate the level of polymorphism among the four canola cultivars. The seven SSR primer sets revealed 31 alleles and 21 out of them were polymorphic (67.7 %). All primers showed different levels of polymorphism except RAM 206, RM 544 and RM 519 which showed no polymorphism among the four canola cultivars (Fig. 2; Table 5). The size of the detected alleles produced from using the SSR primer sets ranged from 75–2000 bp, which reflects a large difference in the number of repeats between the different alleles. These results agreed with Moghaddam et al. (2009) that could successfully assessed the genetic diversity in rapeseed cultivars using RAPD and microsatellite markers.
The AFLP technique has been used for varietal fingerprinting, mapping and genetic diversity studies. The major advantage of the AFLP technique over the other techniques is that it generates a larger number of amplified products in a single reaction (Powell et al. 1996). AFLP’s on average reveal more polymorphic markers then either RFLP or RAPD’s. In B. napus Lombard et al. (2000) indicate that two AFLP primer pair combinations were sufficient to distinguish 83 different cultivars from each other.
In the present investigation, the AFLP analysis was performed using three selective primer combinations and generated a total of 338 bands (Fig. 3; Table 6) The number of markers observed per primer combination ranged from 98 to 130. The total accounting marker number was 338 amplified bands, representing 87 % polymorphism and an average number of polymorphic bands per AFLP primer combination ranged from 79 to 91 bands. The highest percentage of polymorphism was obtained with M-CAT/E-AGG (91 %) followed by M-CAC/E-AGG (90 %) then M-CAG/E-ACC (79 %). The M-CAC/E-AGG produced the highest polymorphic bands (117), while the lowest number was obtained with M-CAG/E-ACC (78). As shown in Table 6, the size of AFLP fragments generated by the different primer combinations ranged from 471–1044 bp and the polymorphic fragments were distributed across the entire size range (Fig. 3; Table 6).
The unique AFLP markers that characterized the four canola varieties are listed in Table 7. Based on the data obtained, it can be distinguished between the four canola cultivars tested according to the AFLP specific markers. The cultivar Semu 249 exhibited the highest number of unique AFLP markers, giving 57 markers, followed by Misser L-16 which was characterized by 40 unique AFLP markers, then Serw-3 with 31 unique markers. While Serw-4 was characterized by the lowest number producing 14 unique positive markers.
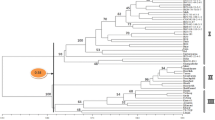
The RAPD; SSR and AFLP based dendrogram group the investigated cultivars into two main clusters. The first cluster included Semu 249, the second cluster is divided into two sub-clusters the first one contains the cultivar Misser L-16 and the second one has the cultivars Serw-3 and Serw-4 (Fig. 4). These results indicate that the cultivars Serw-3 and Serw-4 may possess a high degree of genetic similarity; also their ancestors might be closely related (Fig. 4).
The dendrogram built on the basis of combined data from RAPD, SSR and AFLP analysis represents the genetic distances among the four canola varieties (Fig. 4).
Conclusion
The results of the present study indicate that DNA markers represent efficient tools for estimating the genetic variability and the genetic relationships among the four canola cultivars. The markers generated are enough to distinguish between the different genotypes used. The genotype-specific molecular markers were determined and these markers can be considered as useful markers for high oil production in canola breeding programs. This opens up a possibility for developing a molecular genetic map that will lead to the application of marker-assisted selection tools in genetic improvement of canola.
References
Ahmad N, Munir I, Khan I, Ali W, Muhammad W, Habib R, Khan R, Swati Z (2007) PCR-based genetic diversity of rapeseed germplasm using RAPD markers. Biotechnology 6(3):334–338
Bassam JB, Caetano-Anolles G, Gressho PM (1991) Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem 196:80–83
Basunanda P, Spiller TH, Hasan M, Gehringer A, Schondelmaier J, Lühs W, Friedt W, Snowdon RJ (2007) Marker-assisted increase of genetic diversity in a double-low seed quality winter oilseed rape genetic background. Plant Breed 126:581–587
Charcosst A, Moreau L (2004) Use of molecular markers for the development of new cultivars and the evaluation of genetic diversity. Euphytica 137:81–94
Cheng XM, Xu JS, Xia S, Gu JX, Yang Y, Fu J, Qian XJ, Zhang SC, Wu JS, Liu KD (2009) Development and genetic mapping of microsatellite markers from genome survey sequences in Brassica napus. Theor Appl Genet 118:1121–1131
Cruz VMV, Luhman R, Marek LF, Rife CL, Shoemaker RC, Brummer EC, Gardner CAC (2007) Characterization of flowering time and SSR marker analysis of spring and winter type Brassica napus L. germplasm. Euphytica 153:43–57
Dandelj D, Jakse J, Javornik B (2004) Assesment of genetic variability of olive variaties by microsatellite and AFPL markers. Euphitica 136:93–102
Halton TA, Christopher JT, McClure L, Harker N, Henry RJ (2002) Identification and mapping of polymorphic SSR marker from expressed gene sequence of barley and wheat. Mol Breed 9:63–71
Hassan MB, Sahfique FA (2010) Current situation of edible vegetable oils and some propositions to curb the oil gap in Egypt. Nat Sci 8(12):1–7
Jaroslava A, Polakova K, Leisova L (2002) DNA analysis and their application in plant breeding. Czech J Genet Plant Breed 38:29–40
Kebede B, Thiagarajah M, Zimmerli C, Rahman MH (2010) Improvement of open pollinated spring rapeseed (B. napus) through introgression of genetic diversity from winter rapeseed. Crop Sci 50:1236–1243
Lombard V, Baril CP, Dubreuil P, Blouet F, Zhang D (2000) Genetic relationships and fingerprinting of rapeseed cultivars by AFLP: consequences for varietal registration. Crop Sci 40:1417–1425
Mailer RJ, Wratten N, Vonarx M (1997) Genetic diversity among Australian canola cultivars determined by randomly amplified polymorphic DNA. Aust J Exp Agric 37:793–800
Marijanovic JA, Kondic SA, Saftic-Pankovic D, Marinkovic R, Hristov N (2009) Phenotypic and molecular evaluation of genetic diversity of rapeseed (Brassica napus L.) genotypes. Afr J Biotechnol 8:4835–4844
Ministry of Agriculture and Land Reclamation, Economic Affairs Sector, Central Administration of Agricultural Economics, Food Balance Sheet (2003–07)
Moghaddam M, Mohammmadi SA, Mohebalipour N, Toorchi M, Aharizad S, Javidfar F (2009) Assessment of genetic diversity in rapeseed cultivars as revealed by RAPD and microsatellite markers. Afr J Biotechnol 8(14):3160–3167
Powell W, Morgante M, Andre C, Hanafy M, Vogel J, Tingey S, Rafalski A (1996) The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breeding 2:225–238
Qu C, Hasan M, Lu K, Liu L, Liu X, Xie J, Wang M, Lu J, Odat N, Wang R, Chen L, Tang Z, Li J (2012) Genetic diversity and relationship analysis of the Brassica napus germplasm using simple sequence repeat (SSR) markers. Afr J Biotechnol 11(27):6923–6933
Rogers SO, Bendich AJ (1985) Extraction of DNA from milligram amounts of fresh herbarium and mummified plant tissues. Plant Mol Biol 5:69–76
Sovero M (1993) Rapeseed a new oilseed crop for the United States. In: Janick J, Simon JE (eds) New crops. Wiley, New York, pp 302–307
Spector AA (1999) Essentiality of fatty acids. J Am Oil Chem Soc 34:51–53
Stoutjesdijk PA, Hurlestone C, Singh SP, Green AG (2000) High-oleic acid Australian Brassica napus and B. juncea varieties produced by co-suppression of endogenous Delta 12-desaturase. Biochem Soc Trans 28:938–940
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters JP, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new fingerprinting technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Yang X, Quiros C (1993) Identification and classification of celery cultivars with RAPD markers. Theor Appl Genet 86:205–212
Younessi MH, Nejat AIP, Mohamad T, Abbas M (2011) Phenotypic and molecular analysis of soybean mutant lines through random amplified polymorphic DNA (RAPD) marker and some morphological traits. Afr J Agric Res 6(7):1779–1785
Zeng L, Kwon TR, Liu X, Wilson C, Grieve CM, Gregorio GB (2004) Genetic diversity analyzed by microsatellite markers among rice (Oryza sativa L.) genotypes with different adaptations to saline soils. Plant Sci 166:1275–1285
Acknowledgments
The authors express deep sense of gratitude to the Field Crop Institute, Agricultural Research Center, Ministry of Agriculture–Egypt, for providing the canola seed used in this study. Also for the staff member of the Genetic Engineering Research Center, Cairo University for all the support, assistance and constant encouragements to carry out this work.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Moghaieb, R.E.A., Mohammed, E.H.K. & Youssief, S.S. Genetic diversity among some canola cultivars as revealed by RAPD, SSR and AFLP analyses. 3 Biotech 4, 403–410 (2014). https://doi.org/10.1007/s13205-013-0165-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13205-013-0165-x