Abstract
γ-Al2O3 was synthesized by the Sol–gel method, Ni (NO3)2 was placed in the pores by the impregnation method, and Ni-γ-Al2O3 was obtained by pyrolysis in a hydrogen stream in a CVD device. By the method of chemical vapors phase deposition (CVD) on Ni-Al2O3 catalytic converter with decomposition of methane in the natural gas produced carbon nanotubes (CNT) (Chunduri et al. in Mater Express 4(3):235–241, 2014; Zhou et al. in Appl Catal B 208:44–59, 2017). The catalytic activity of the catalysts in methane decomposition was examined from 650 °C to 900 °C by the method of chemical vapors phase deposition (CVD), the yield of CNTs tends to increase with the growth at the ratio of natural gas supply to hydrogen. The specific surface increases with an increase of nickel content and can reach 265.5 m2/g for a sample of 2% Ni-A12O3 at 850 °C. Growth at the temperature of methane decomposition leads to reduction in its specific surface. It has been established that the use of the Ni-Cu/γ-Al2O3 catalytic system, in which copper acts as a stabilizing additive, makes it possible to double the maximum yield of the carbon product during the decomposition of natural gas.
Similar content being viewed by others
Introduction
One of the traditional ways to carry out the endothermic reaction of decomposition of methane to carbon and hydrogen is high-temperature pyrolysis, which runs through the reaction:
The advantage of this method is the complete absence of gaseous pollutants in the form of CO and CO2 in the mixture, with the exception of methane, which is under compressed, which is easily separated from H2 due to sharply different physical and chemical characteristics. It should be noted that the carbon materials produced by the reaction are very valuable products. They can be used in the production of sorbents, catalyst carriers, fixed chromatographic phases, various composite materials, pigments, etc. Their properties such as mechanical elasticity and noticeable electrical conductivity imply the use of such materials in the electronics industry. Metals of 4th periodical system of 8th groups elements such as iron, nickel and cobalt are more active in the methane decomposition reaction Methane catalytic activation is an important reaction step in many chemical processes, particularly in methane cracking, and Chemical Vapor Deposition (CVD), in which hydrogen and carbon nanotubes (CNT) or nanofibers (CNF) are formed [3,4,5,6,7,8,9]. Methane is also the main component of natural gas and used to synthesize CO and hydrogen, via reforming processes (steam reforming, partial oxidation, dry reforming, or combination thereof). The hydrogen produced by such reforming processes is mixed with large amounts of CO, which may limit its applicability as a potential power source. For example, in proton exchange membrane (PEM) fuel cells technology, even a small trace of CO (typically above 10 ppm) will poison the electro-catalysts in the fuel cell [10, 11]; thus, expensive H2 purification process is needed, making methane cracking an attractive alternative for the production of CO-free hydrogen. Methane cracking consists in breaking methane into molecular hydrogen and carbon. Experimental observations have shown that methane cracking is unlikely to happen (within reasonable time) at temperatures below 1000 °C without the presence of a catalyst. However, catalytic cracking of methane can occur at temperatures between 500 and 800 °C [12]. Among the different transition metals commonly used in catalytic methane cracking, Ni-based supported catalysts show very good catalytic activity at relatively moderate temperatures [3, 4, 13,14,15]. The most frequently used supports are SiO2, Al2O3, and MgO [3, 15].
Methane decomposition over transition metals such as Ni, Fe or Co, is a promising approach for CNT synthesis due to the abundance and low cost of natural gas. Among the catalysts, Ni is characterized by exhibiting the highest catalytic activity in the production of CNTs [6, 10].
Experimental part
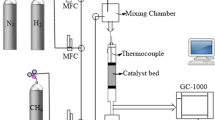
Carrier-nano γ-Al2O3 is obtained as follows: Al(NO3)3-9H2O was dissolved in distilled water, after that monoethanolamine in Al3+/MEA = 1:2 ratios was added to the reaction mixture. Next, the mixture was mixed and ashing of the solution took place at 40 °C within 60 min. Then the temperature of the medium was raised to 90 °C, at this temperature the ashes turned into gel. The resulting precipitated gel was separated by filtration, washed 5 times with various solvents (water, alcohol) by replacing them with fresh portions each time. The gel obtained after drying was transferred to the crucible and subjected to calcination in the furnace at 850 °C until the cessation of weight loss. Activation of γ-Al2O3 was carried out at the chemical vapor deposition unit (CVD) at 400 °C for 2 h and at 750 °C for 4 h in the flow of argon with hydrogen at the ratio of 80:20% (vol.). The size of crystals obtained by γ-Al2O3 according to the data of diffraction maximums was 4–6 nm. Then Ni(NO3)2 was applied to γ-Al2O3 by impregnation and dried for 2 h at 200 °C. Catalyst recovery was carried out on CVD at 480–500 °C by passing for 1 h through a quartz tube at a rate of 500 ml/min diluted in argon hydrogen (20 vol.%). Slow reduction of Ni(NO3)2 allowed us to obtain highly dispersed metal nanoparticles on the surface of γ-Al2O3.
Nanopowders of aluminum and nickel hydroxides were synthesized for welding to 2 M solution of Al(NO3)3∙8H2O and 0,05–0,25 M solution of Ni(NO3)2 separately in deionized water and a stabilizer of monoethanolamine ashes (MEA) was added with the molar ratio of MEA/Al(Ni) = 2 at 300 rpm mixing speed at room temperature for 1 h. Then by increasing temperature to 95 °C, synthesized ashes were brought to the gel state and the colloid solution were treated with ultrasound 26 MHz for 10 min, after that they were repeatedly neutralized with distilled water, filtered, evaporated at 100 °C and then calcined in CVD at 850 °C for 4 h.
Hydrogen is used in the process of CNTs synthesis to activate the catalyst in the reaction mixture [18], which leads to the formation of active metal particles [16, 17]:
Results and discussion
Natural gas was exposed to pyrolysis in the presence of synthesized catalysts for producing CNTs. As it is obtained from the results of these experiments, the mode of activation of the catalyst in hydrogen allows to get significantly higher yield of carbon product for the same time of the process (Fig. 1).
At the next stage, the influence of reaction mixture composition on carbon product yield was studied. It is shown that partial dilution of reaction mixture by hydrogen allows to prolong time of active work of the catalyst and, as a consequence, leads to increase in output of carbon product that in ~ 5 times exceeds value received at decomposition of undiluted gas (Table 1).
Table 1 shows that as the increase of pyrolysis temperature from 650 to 900 °C, the yield of nanocarbon grows. So, the yield of CNTs tends to increase with the growth at the ratio of natural gas supply to hydrogen. However, decomposition of carbon containing gas can also occur in the gas phase with the formation of amorphous carbon which slows down the growth of CNTs while depositing on the catalyst. As it is observed at the figure number 3, when the medium temperature rises up to 900 °C due to decontamination of the catalyst, the output of nanocarbon stops. Therefore, it is necessary to note the special role of hydrogen in CNTs cultivation within the process of hydrocarbon decomposition. During the discussion of results about the impact of H2 on the growth rate of CNTs, two opposite trends should be taken into account. Firstly, the process is related to the ability of hydrogen. So, Hydrogen controls the deposition of amorphous carbon blocks the active surface of the catalyst and reacts with carbides, also amorphous carbon deposits. Additionally, this procedure relates with removing of them from the place of growth of carbon nanotubes that lead to an extension of its operating time. On the other hand, an increase in the concentration of hydrogen leads to the growth in the contribution of hydrogenation reaction of the carbon which leads to a decrease in the observed rate of CNTs accumulation.
Analysis of the effects of temperature and hydrogen content in the gas mixture which is fed into the reactor on the nature of carbon nanostructures allows us to conclude that their formation and growth on the catalyst Ni-Al2O3 proceeds in accordance with the decomposition–diffusion–deposition mechanism. The source molecule of hydrocarbon decomposes on the surface of a catalytic particle with a successive separation of H from the chemosorbed CHm fragment. Then the carbon atoms diffuse towards another catalytic surface where the carbon nanotubes grow.
Further, the regularities of carbon nanostructures production from the catalyst quantity, temperature, composition of the reaction mixture and the method of applying the catalyst on the carrier substrate were investigated.
Figure 2 shows the kinetic curves of CNTs accumulation depending on the amount of applied active component.
Two characteristic areas can be identified on the kinetic curve: the growth period of CNTs and the catalyst decontamination. Decontamination of the catalyst is caused by amorphous carbon deposition on the surface of active particles which leads to their blocking.
This graph also shows that as an increase at the content of catalyst, the time of its active work raises and it in turn leads to a corresponding growth in the output of the CNT. The data obtained represents concentration of applied catalyst which is equal to 2.0% wt. Ni is optimal for the effective use of active component, since with a relatively small number of active centers, the output of CNTs can be controlled in a fairly wide range of values, varying the duration of natural gas decomposition process. The texture properties of an CNT are determined with the help of composition of the catalyst. Thus, the specific surface increases with an increase of nickel content and can reach 265.5 m2/g for a sample of 2%Ni-A12O3 at 850 °C. Growth at the temperature of methane decomposition leads to reduction in its specific surface.
When the optimal temperature is assigned during synthesis, the process temperature varied from 550 to 900 °C. From the kinetic data presented in Fig. 3, it can be seen that at 900 °C the rate of formation of carbon deposits is very high and the catalyst decontamination is fast too. To conduct the process at temperature close to 900 °C is undesirable, as it leads to the partial formation of a further cubic modification of α-Al2O3 and adversely affects the output of CNTs[19].
On the other hand, decrease in the reaction temperature to 550 °C leads to a sharp decline in the yield of carbon product compared with the results obtained at 700 and 850 °C (4.5 and 9.5 times, respectively). Thus, for further experiments 850 °C was chosen as process temperature at which it is possible to control the output of product in a sufficiently wide range of values.
It was found that the outputs of CNTs on catalysts prepared by various methods differ from each other (Table 2).
It was found that the usage of the catalyst obtained by sol–gel method, the yield of product becomes significantly higher compared to the yield of it in the presence of a catalyst prepared by impregnation with nickel nitrate, at the same time they are characterized by longer duration of operation (up to 15 min) Thus, the sol–gel method provides a high yield of carbon product with longer catalyst activity.
An equally important parameter that affects the yield and structural type of carbon product is the composition of catalyst. It is known that bi- and three-component catalysts for the synthesis of dispersed CNTs such as Ni-Cu, Fe-Mo, Co-Mo are more stable and productive in the hydrocarbon decomposition reaction [20, 21]. The choice of copper as a promotion additive was due to several reasons. First of all, copper is easily mixed with nickel, forming alloys throughout the concentration range. Secondly, the addition of copper to a nickel catalyst increases the yield of carbon product and also leads to changes in the structure of carbon yarns [22].
The results of the study showed that the regularities characteristic of dispersed systems are also preserved in case of hybrid materials synthesis (Fig. 4). Thus, the introduction of 20 wt% of copper causes a two-fold increase in CNTs output due to increase of the catalyst active time (from 12 to 18 min). It should be noted that during the first minutes of reaction, decomposition rate of hydrocarbons is slightly higher in the case of monometallic catalyst (Fig. 4, curve 2).
The results obtained can be explained by the fact that the addition of inactive copper enriches the surface of catalyst particles, stabilizing its operation and due to the reduction of coke-causing ability there is an increase in the time of the catalyst active operation.
It is shown that the mode of preactivation of the catalyst in hydrogen is crucial for obtaining a high yield carbon product. Introduction of hydrogen into reaction mixture allows to prolong time of catalyst active work and as a result, to obtain higher yield of carbon product.
It was found that the sol–gel catalyst synthesis method provides a high yield of CNTs at longer catalyst activity.
The use of a catalytic system, where copper acts as a stabilizing additive, allows doubling the maximum yield of carbon product in the decomposition of natural gas.
Mechanism of CNT formation
The proposed mechanism of CNT formation is schematically represented in Fig. 5. The characteristic ability of metal catalyst particles to form the ordered carbon is believed to be related to their catalytic activity for the decomposition of carbon compounds, and that carbon is able to diffuse through and over the metals rapidly. The property allows the ordered carbon to be produced by a diffusion and deposition mechanism. It also means that the graphite structure is formed only in the vicinity of the metal particle surface, and the amorphous carbon may be produced if there is a significant reaction away from the metal. The surface reaction, that is CH4 cracking, produces the pyrolytic carbon atoms. The isolated surface pyrolytic carbon atoms that are important in the formation of CNTs are dissolved into Ni-particle on the gas side, which creates a selvedge with high concentration because of the segregation behavior of carbon at the front of Ni-particle. Subsequently, the pyrolyzed carbon atoms are incorporated into the active crystal surface of the catalyst particle to diffuse through it, and finally deposit on the other side of the catalyst particle to form CNTs. In the initial stage of CVD, the gaseous hydrocarbon, CH4, has a faster decomposition rate, because the catalyst surface active sites are not completely occupied by the pyrolytic carbon atoms. Simultaneously, the selvedge with high concentration of pyrolytic carbon atoms creates a larger concentration gradient resulting in a fast diffusion rate. The growth process of CNTs can be specifically described as the following three stages:
When the catalyst-substrate interaction is weak (metal has an acute contact angle with the substrate), hydrocarbon decomposes on the top surface of the metal, carbon diffuses down through the metal, and CNT precipitates out across the metal bottom, pushing the whole metal particle off the substrate. As long as the metal’s top is open for fresh hydrocarbon decomposition, the concentration gradient exists in the metal allowing carbon diffusion, and CNT continues to grow longer and longer. Once the metal is fully covered with excess carbon, its catalytic activity ceases and the CNT growth is stopped.
Conclusion
The main results can be summarized:
-
Introduction of hydrogen into the reaction mixture allows prolonging the time of the catalyst active operation and, as a result, obtaining a higher yield of carbon product.
-
It was found that the synthesis of Ni/γ-Al2O3 sol–gel catalyst by method provides high yield of UNT at longer catalyst activity.
-
The use of the catalytic system Ni-Cu/γ-Al2O3, where copper acts as a stabilizing additive, allows doubling the maximum yield of carbon product in the decomposition of natural gas.
References
Chunduri LA, Rattan TM, Molli M, Kamisetti V (2014) Single step preparation of nano size gamma alumina exhibiting enhanced fluoride adsorption. Mater Express 4(3):235–241
Zhou L, Enakonda L, Harb M et al (2017) Fe catalysts for methane decomposition to produce hydrogen and carbon nano materials. Appl Catal B 208:44–59
Hou K, Hughes (2001) The kinetics of methane steam reforming over a Ni/α−Al2O3 catalyst. Chem Eng J 82:311–328
Fonseca A, Assaf EM (2005) Production of the hydrogen by methane steam reforming over nickel catalysts prepared from hydrotalcite precursors. J Power Sources 142:154–159
Chen L, Qi Z, Zhang S (2020) Catalytic hydrogen production from methane: A review on recent progress and prospect. Catalysts 858:1–18
Yamada Y, Hosono Y, Murakoshi N, Higashi N, Ichi-oka H, Miyake T et al (2006) Carbon nanofiber formation on iron group metal loaded on SiO2. Diam Relat Mater 15(4–8):1080–1084
Romero A, Garrido A, Nieto-Marquez A, Sanchez P, de Lucas A, Valverde JL (2008) Synthesis and structural characteristics of highly graphitized carbon nanofibers produced from the catalytic decomposition of ethylene: influence of the active metal (Co, Ni, Fe) and the zeolite type support. Microporous Mesoporous Mater 110(2–3):318–329
Takenaka S, Shigeta Y, Tanabe E, Otsuka K (2003) Methane decomposition into hydrogen and carbon nanofibers over supported Pd-Ni catalysts. J Catal 220:468–477
Li Y, Li D, Wang G (2011) Methane decomposition to Cox-free hydrogen and nano-carbon material on group 8–10 base metal catalysts: a review. Catal Today 162:1–48
Haryanto A, Fernando S, Murali N, Adhikari S (2005) Current status of hydrogen production techniques by steam reforming of ethanol: a review. Energy Fuels 19:2098–2106
Matsumura Y, Nakamori T (2004) Steam reforming of methane over nickel catalysts at low reaction temperature. Appl Catal A Gen 258:107–114
Wei J, Iglesia E (2004) Isotopic and kinetic assessment of the mechanism of methane reforming and decomposition reactions on supported iridium catalysts. Phys Chem Chem Phys 6:3754–3759
Laosiripojana N (2005) Assabumrungrat. Methane steam reforming over Ni/Ce−ZrO2 catalyst: Influences of Ce−ZrO2 support on reactivity, resistance toward carbon formation, and intrinsic reaction kinetics. Appl Catal A Gen 290:200–211.
Jones G, Jakobsen JG, Shim SS et al (2008) First principles calculations and experimental insight into methane steam reforming over transition metal catalysts. J Catal 259:147–160
Blaylock DW, Ogura T, Green WH, Beran GJO (2009) Computational investigation of thermochemistry and kinetics of steam methane reforming on Ni (111) under realistic conditions. J Phys Chem C 113:4898–4908
Lamacz A (2019) CNT and H2 production during CH4 decomposition over Ni/CeZrO2 I: A mechanistic study. Chem Eng 3:1–16
Zhi-hui Hu, Dong S-M, Jian-bao Hu, Wang Z, Bo Lu, Yang J-S, Li Q-G, Bin Wu, Gao Le, Zhang X-y (2012) Synthesis of carbon nanotubes on carbon fibers by modified chemical vapor deposition. New Carbon Mater 27:352–361
Ibrahimov HD, Amirov FA, Huseynov HJ, Ibragimova ZM, Zamanova LS, Asadzadeh RN, Jabarov SH (2019) Carbon nanotubes obtained from natural gas by CVD. J Surf Invest 13:1244–1247
Zhan S, Tian Y, Cui Y, Wang Y, Ye S, Chen Y (2007) Effect of process conditions on the synthesis of carbon nanotubes by catalytic decomposition of methane. China Particuol 5:213–219
Jourdain V, Bichara C (2013) Current understanding of the growth of carbon nanotubes in catalytic chemical vapour deposition. Carbon 58:2–39
Daniel T, José L, Isabel S (2018) Co-, Cu- and Fe-doped Ni/Al2O3 catalysts for the catalytic decomposition of methane into hydrogen and carbon nanofibers. Catalysts 8:1–15
Downs WB, Baker RTK (1991) Novel carbon fiber-carbon filament structures. Carbon 1991(29):1173–1179
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahimov, H., Malikli, S., Ibrahimova, Z. et al. Ni-γ-AL2O3 catalysts for obtaining nanocarbon by decomposition of natural gas. Appl Petrochem Res 11, 123–128 (2021). https://doi.org/10.1007/s13203-021-00264-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-021-00264-0