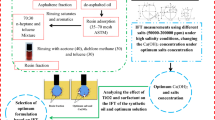
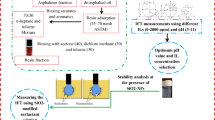
Abstract
Unfortunately, oil reservoirs are complex considering the fluids (e.g., crude oil composition) and rock properties making it hard to propose a simple enhanced oil recovery (EOR) method for higher oil production. Besides, most of the investigations had focused on crude oil which is a complex mixture of thousands of components making it hard to extract any reliable conclusions with respect to the crude oil type. So, the current study is focused on the application of ionic liquids from different families of pyridinium and imidazolium, titanium oxide nanoparticles, and salts (MgCl2 and CaCl2) in the presence of resinous synthetic oil for the first time. The obtained results using the central composite design (CCD) approach revealed the positive effect of resin fraction on the IFT reduction by 27% considering the initial value (34.9%). Using the CCD approach revealed that using pH = 7, MgCl2 concentration = 21,000 ppm, CaCl2 concentration = 21,000 ppm, resin fraction of 9wt%t and 500 ppm of [C12mim][Cl] concentration reduces the IFT to minimum value of 0.62 mN/m while the minimum IFT value for optimum conditions of solution includes [C12py][Cl] led to minimum IFT value of 2.2 mN/m. But, the contact angle measurements revealed better synergy between [C12py][Cl] and TiO2-NPs (0–200 ppm) for better wettability alteration toward water-wet condition (27.3°) than [C12mim][Cl] (33.2°). Moreover, the IFT measurements revealed that the presence of TiO2-NPs is effective in reducing the IFT of the optimum formulations to 0.55 and 0.84 mN/m for [C12mim][Cl], and [C12py][Cl], respectively. According to the results, it seems that the obtained optimum formulations for [C12mim][Cl], and [C12py][Cl] are applicable for EOR purposes as new hybrid solutions.
Similar content being viewed by others
Introduction
Using enhanced oil recovery (EOR) processes increased over the past three decades due to the sources of energy limitations and the oil production reduction while the consumption increases daily facing the entire globe with energy crises. In detail, with the aid of the reservoir's natural pressure, only 10–15% of the original oil in place (OOIP) can be produced means the necessity of performing secondary and tertiary oil recovery processes to extract the trapped oil in the reservoir. In detail, it is possible to produce 10–15% in addition to the primary oil production through the secondary oil recovery processes. So, even after secondary oil recovery processes, more than 65–70% of the OOIP remained unrecovered which is a significant amount of wealth that remained trapped and unrecovered.
At this point, it is highly required to use new and innovative oil recovery processes commonly known as EOR methods such as gas injection, chemical injection, in situ combustion, modified-water injection (such as smart water and low-salinity water), microbial injection, and nanoparticles injection to extract the remained oil in the reservoir. Unfortunately, although several EOR approaches introduced and examined over the past years, the main shortcomings of the tested EOR methods are their limitation in activating activate a few mechanisms. Respecting these limitations, a combination of several EOR methods have been proposed as hybrid methods during the past decade to activate multiple mechanisms with the main concentration on the IFT reduction, wettability alteration, viscosity reduction, and swelling of crude oil (Cheraghian 2015; Abhishek et al. 2015).
In other words, considering the energy crises, and limitations of each EOR method, it is highly required to use hybrid methods (Cheraghian and Hendraningrat 2016). As a way out, hybrid methods were proposed based on the concomitant application of the low-salinity aqueous solution, surfactant solutions, nanoparticles (NPs), etc. (Bera and Belhaj 2016; Khalil et al. 2017; Li et al. 2021; Negin et al. 2016). Among the EOR methods, nanoparticle injection is one of the new approaches which can reduce the IFT, wettability of the rock surface toward water-wet status, reduce crude oil viscosity (Ju et al. 2006; Torsater et al. 2012; Zaid et al. 2013; Al-Anssari et al. 2016; Saien and Gorji 2017; Ehtesabi et al. 2015; El-Diasty and Ragab 2013; Kazemzadeh et al. 2015; Mohammadi et al. 2017; Taborda et al. 2016, 2017; Wei et al. 2007).
Besides the aforementioned possible mechanism, it is also possible to produce nano-emulsion using NPs (Bobbo et al. 2012) for selective plugging the pores (Anganaei et al. 2014; Hashemi et al. 2013), thermal conductivity enhancement purposes (Aveyard et al. 2003; McElfresh et al. 2012) disjoining pressure manipulation (McElfresh et al. 2012; Zamani et al. 2012).
The other important feature of the NPs is their carrying capability which can transport the compartments to the narrow pathways and small pores where the oil droplets are trapped. In light of this capability, NPs are good candidates to be used as a carrier to deliver specific chemicals to a specific destination in the pore structures and networks (Rodriguez et al. 2009). Considering these unique advantages of NPs, many researchers focused on the application of NPs in the EOR processes to recover more trapped oil by activating two main mechanisms of IFT reduction and wettability alteration besides the other mechanisms aforementioned (de Castro Dantas et al. 2017; Moradi et al. 2015; Suleimanov et al. 2011).
As an example, the effect of polysilicon NPs (PSNP) was investigated in changing the rock wetness since it is a vital mechanism especially if it moves toward strongly water-wet conditions (Onyekonwu and Ogolo 2010). Among the different possible NPs, it is well established that using silica-based NPs is a good approach to move the wettability of the rock surfaces toward strongly water-wet (highly required to produce more trapped oil under constant rate) (Cheraghian 2016; Hendraningrat et al. 2013b, c, d; Torsater et al. 2012).
The point is that although silica-based NPs are potential NPs for EOR purposes, there are other NPs examined by different researchers such as Al2O3 and iron oxide (Fe2O3). In detail, Mohammadi et al. (2014) performed several experiments to find if Al2O3-NPs are capable of producing higher oil from the sandstone rocks. Just a year after, Tarek (2015) investigated the idea of using a mixture of different NPs including Al2O3, iron oxide (Fe2O3), and SiO2 to prepare a hybrid solution for tertiary oil recovery purposes which can activate different effective mechanisms and enlighten this fact that the NPs are potential to modify the thermal conductivity; but also they are potential to activate several effective mechanisms such as IFT reduction and wettability alteration (Cheraghian and Hendraningrat 2016; Sheng 2010).
Similar to the NPs considered new and innovative chemicals for EOR purposes, ionic liquids (ILs) which are a new class of surfactants that proposed due to their unique features. In detail, ILs are a new class of chemicals with numerous characteristics such as being highly stable under harsh salinity and temperature conditions (salinity and temperature) (Chen et al. 2014; Dharaskar Swapnil 2012; Domańska 2005; Lee and Kim 2013; Martins et al. 2014; Peng et al. 2011). These characteristics of ILs come from their unique and bulky structure since they are generally comprised of cationic and anionic sections making it possible to fabricate any specific-task IL regardless of the application.
In detail, it is possible to fabricate any desired IL for any specific purpose such as EOR processes with tailoring proper cationic and anionic sections (José-Alberto and Jorge 2011; Khupse and Kumar 2010). For example, different researchers have investigated the possible application of different ILs, especially from the imidazolium family for EOR purposes through reservoirs with harsh salinity (Hezave et al. 2013b; Smit et al. 1991).
The results reported by those researchers revealed that the dissolution of cationic ILs in the aqueous solution can neutralize the opposite charges that existed in the solution due to the dissolved salts providing the chance of easier accumulation of surfactant molecules in the interface which means lower IFT values. The point must be clarified that the results reported by Hezave et al. (2013a) demonstrated that this is not only the IFT reduction leading to higher oil recovery during the EOR processes, but also this is the concomitant effect of IFT reduction and wettability alteration causes more oil production (Rodríguez-Palmeiro et al. 2015).
The other point is that besides the chemical combination, there is a complicated and undeniable interaction between the crude oil type and the chemical combination concomitant with the operating conditions such as pH (Demirbas 2016; Demirbas and Taylan 2015; Muhammad et al. 2013).
In general, crude oil is comprised of saturates, aromatics, resin, and asphaltene fractions, and the last two fractions are the most essential because of their detergency nature which can act as natural surfactants (Demirbas et al. 2015; Lashkarbolooki et al. 2014). So, several investigations were focused on the possible interactions that exist between the chemicals and these fractions acting as the natural surfactant and consequently affect the wettability and IFT (Andersen 1994; Lashkarbolooki et al. 2016; Mozaffari 2015; Mozaffari et al. 2021, 2015; Wu et al. 1998).
Considering the existed ample space for the interactions between a new class of IL-based surfactants from pyridinium and imidazolium families in the presence of NPs and since there is a small number of investigations dealing with specific fractions of crude oil, especially resin, and asphaltene fractions, performing this investigation is essential for EOR purposes. The point is that using specific fractions for IFT reduction and wettability alteration investigations is required since these fractions can act as natural surfactants and introduce interactions with other chemicals such as surfactants and alkalis. Moreover, most of the performed previous investigations used changing one factor at a time as the design of the experiment approach. In contrast, in the current investigation, central composter design (CCD) is used which provides the chance to investigate the possible interactions between several parameters on the IFT reduction and wettability alteration. To sum up, this investigation is essential since the impacts of several parameters including IL type, pH, salt type, and low and high salinity conditions in the absence and presence of metallic-based NPs of TiO2 on the wettability alteration and IFT reduction were examined for the first time. In this way, the present work is designed in a way to examine the application of synthetic resinous oil prepared from the resin fraction of heavy-acidic crude oil, pyridinium, and imidazolium-based ILs of [C12py][Cl], and [C12mim][Cl] and TiO2-NPs under different concentrations of 0–1000 ppm and 0–200 ppm, respectively, for IFT reduction and wettability alteration purposes at different pH values of 3–11 for the first time based on the best knowledge of the authors. Besides, instead of using formation brine which is a complex combination of different salts, two divalent salts of MgCl2 and CaCl2 with low and high salinity conditions (1000–41000 ppm) were selected as the target salts to prepare the sample brine.
The worth mentioning point is that using NPs for EOR purposes has risk which is the risk of NPs precipitation due to agglomerations or other phenomena that may lead to pore plugging which in severe cases can cause catastrophic outcomes which are entrapment of crude oil in the reservoir forever. In light of this limitation, it is highly required to investigate the stability of TiO2-NPs in the presence and absence of ILs to ensure the long-term stability of the prepared solutions to unveil the possible risk of TiO2-NPs precipitation.
The other point is that the current investigation mainly focuses on applying resinous synthetic oil with concentrations of 0–9 wt% dissolved in toluene. This range of concentration was selected since it can cover the resin concentration in different crude oils since it is merely passing 10 wt% in crude oils. Also, the obtained results can be used for the resin fractions structurally similar to the examined resin fraction (considering the aromaticity). Moreover, the main goal of this selection is to characterize the impact of the used chemicals on the resin fraction which is one of the most essential fractions in crude oil. Generally, crude oil is a combination of thousands of compounds that can be categorized into four general fractions saturates, aromatics, resin, and asphaltene (SARA analysis). In this way, using a specific fraction to prepare the synthetic oils provides a better chance to differentiate between the impacts of chemicals on each fraction for more reliable conclusions. After that, by knowing the impact of chemicals on the different fractions, anyone can find the effect of examined chemicals on the crude oils using the SARA analysis. In other words, in the light of knowing each fraction's role in the efficiency of the used chemical, one can extract generalized conclusions without performing a large number of experiments. Unfortunately, this approach has a limitation that comes from the different structures of each fraction, especially resin and asphaltene fractions. However, the point is that it is possible to eliminate this limitation to some extent if researchers use some identification such as aromaticity to characterize the existing fractions in the crude oil.
Materials and methods
Material and solutions
The sample crude oil which is used to extract resin fraction was a heavy-acidic crude oil with asphaltene and resin contents of 6.3% and 9.8%, respectively, which was provided by one of the Iranian oil companies (density of 0.923 g cm−3 @ ambient temperature (dorud oilfield)). This oilfield is the largest Iranian offshore oil field (25 km and 5 km long and wide, respectively) located in the Persian Gulf with 88 wells, 47 are production wells (Chehrazi et al. 2013a). The formation of this oilfield is a combination of Asmari, Surmeh, Yamama, and Manifa which the Asmari section has an APIo degree of 23–25 while the APIo of Surmeh, Yamma, and Manifa are 29.5, 35, and 31, respectively (Setudehnia 1978). The worth mentioning point is that the current oilfield has 2 gas injection wells, and 12 water injection wells which means that this oilfield is a good candidate for the water-based EOR processes. On the other side, the maximum production of 139.74 thousand bpd of crude oil and condensate was the old record of this reservoir which will lose its economic production rate in 2041. Respecting the Dorood structure, it can be observed that this oilfield is an elongated anticline, plunging toward the NNE from Cretaceous and late Miocene times. In detail, two independent structural events from late Cretaceous times led to an NNE–SSW-orientated folds while the second structural event occurred in late Oligocene–Pliocene times which uplifted the structure (Bosold et al. 2005; Chehrazi et al. 2013b; Fard et al. 2006).
The point is that during the first period of structural modification, WNW–ESE which was perpendicular to the present-day fold axis was the main directional movement. On the other hand, the main stress direction during the second structural event was NE–SW, with a slight change to N020 (Berberian and King 1981; Beydoun 1991; Ghazban 2007; James and Wynd 1965; Setudehnia 1978).
The required salts of MgCl2 and CaCl2 were supplied from Sigma-Aldrich, the USA, with a minimum purity of 99.0%, and the TiO2 nanoparticles (20–30 nm) were prepared from Borhan, Iran, with a minimum purity of 99%. Besides, the required ILs were synthesized using a standard procedure and materials using 1-methyl pyridinium, 1-chloro dodecane, and 1-chloro octane (Merck/Fluka, purity > 99.5%).
Interfacial tension measurement
The required IFT and contact angle values were measured via a pendant drop equipment works based on the pendant drop mechanism which is one of the most accurate methods of IFT and contact angle measurements (Yang et al. 2014). In this way, ambient condition pendant drop equipment was used in the present study (APEX Technologies Co., Arak. Iran).
A brief description of the used equipment and related theory is give n by Stauffer (1965).
where Δρ, g, and H are the difference between the bulk and drop phases, acceleration of gravity, and the shape-dependent parameter, respectively. Besides, the H value in Eq. 1 known as the shape factor is correlated to the S value which is the d/D. To calculate the S value, it is required to find the D and d diameters which are the equatorial diameter and the diameter at the distance D from the top of the drop, respectively. The equipment has three sections (a) an automatic dispensing system, (b) an image recording, and (c) a dispatching section, and online IFT and contacts angle calculation software. Using the first section, the required drop volume can be dispensed at the tip of the nozzle in an upward or downward direction depending on the difference between the bulk phase and drop phase using an automatic micro-metering injection system. In the second section, the camera and lens assembly capture the required images at different time intervals and dispatch them to the calculation software where the images are analyzed and the small diameter and large diameters are detected and calculated, and then converted to the IFT values. The point that must be defined is that the used software automatically detects the small and large diameters and then converts the calculated parameters to the IFT values.
Contact angle measurement
Knowing the wettability of a system is one of the most essential characteristics of several industries from enhanced oil recovery processes to medical. In this way, during the past decades, several methods have been proposed to measure the wettability of the systems one of the most essential methods is contact angle measurement. Among the different methods for measuring the contact angle values, using the sessile drop is the most widely used and accurate method which provides the chance of static CA measurement for the operator with an acceptable level of accuracy using online software. If the water CA is lower than 90°, the surface is said to be hydrophilic, and if the contact angle is higher than 90°, the surface is hydrophobic.
Resin extraction procedure
In this study, IP 143/90 (Petroleum, 1985) was used to extract the asphaltene fraction in the first stage and then the resin extraction was isolated from the de-asphalted crude oil. The point is that among the different fractions of the crude oil, resin, and asphaltene fractions is a natural surfactant that has significant effects on the IFT reduction during the chemical EOR methods. It is required to investigate the sole and combinative effect of these fractions in the presence of different chemicals. Besides the effects of these fractions on the possible IFT or wettability alteration, crude oil is a combination of thousands of chemicals makes it hard to extract any reliable and generalized conclusion if the crude oil is being studied for EOR purposes. Respecting these reasons, several investigations were performed to study the role of different chemicals in the presence of only resin and asphaltene fractions (Lashkarbolooki et al. 2014, 2016; Wu et al. 1998) leading to no generalized and consistent results. In brief, using the IP 143/90 for asphaltene isolation purposes, n-heptane with a ratio of 40:1 was applied in the first place. Then, the remaining molten and de-asphalted oil was contacted with a silica gel column (Merck, 35 − 70 mesh ASTM) to extract the resin fraction using the column chromatography method (Soorghali et al. 2014). After that, saturates and aromatics were washed and eliminated from the extracted fraction using 70:30 n-heptane and toluene solution. At last, an acetone/dichloromethane/toluene mixture with a ratio of 40:30:30 was used to achieve the resin fraction (Yarranton et al. 2000).
Besides the isolation of resin and asphaltene fraction, these two fractions were elementally analyzed using a CHNSO analyzer (Thermo Flash EA 1112 series) to determine the C, H, N, S, and O contents. Based on the performed analysis, the resin fraction with the H/C ratio of 1.39 compared with the asphaltene H/C ratio of 1.17 revealed lower aromaticity characteristics which means a more branched structure of resin fraction than the asphaltene molecules. With respect to this finding, it seems that the resin molecules are more similar to the surfactant molecules than the asphaltene molecules.
Central composite design (CCD) approach
Using the design of the experiment approach to lower the required number of experiments is one of the most promising methods used during the past decades. These types of approaches are highly applicable, especially for systems dealing with large numbers of independent parameters and wide ranges of intervals. In other words, using the design of the experiment approach except changing one factor at a time substantially reduces the required number of experiments. Among the widely used and examined design of experiment approaches, central composite design (CCD) under response surface methodology is among the widely used methods. In this way, the CCD approach was used to examine the effect of different operating parameters such as pH, IL type and concentrations, salinity level (low and high salinity conditions) and salt type (CaCl2 and MgCl2), and resin concentrations, while IFT was selected as a response. In more detail, a CCD approach was applied to optimize the variables to obtain the best responses. So, five levels of − α, − 1, 0, + 1, and + α were selected for each factor as the lowest, low, center, high, and highest levels, respectively. All experimental designs were executed randomly to minimize experimental errors precisely. Then, the analysis of variance ANOVA from Design Expert 7.0 software with a 95% confidence level was used to investigate the contribution of main factors and their interactions.
Results and discussions
Resin fraction and interfacial tension
In the first stage of this investigation, the effect of resin fraction was investigated on the IFT reduction if it dissolves in the toluene. The performed CHNSO test (Thermo Flash EA 1112 series) revealed that the resin fraction has H/C ratio of about 1.39 while the asphaltene H/C ratio was 1.16 means the lower aromaticity of the resin fraction consequently leading to a more branched structure of resin. As a consequence of this type of structure, it seems that extracted resin fraction from the heavy-acidic crude oil introduces a more surfactant nature compared with the asphaltenic fraction. On the other side, since the main focus of the current investigation is the interactions between the chemicals and operating conditions with the resinous synthetic oil, no further analyses were performed on the asphaltene fraction in this study. In the first place, the effect of resin concentration was examined in the range of 1–9 wt% to find if this fraction can act as a surfactant. In this way, four IFT measurements using distilled water were performed with resin concentrations of 0, 1, 5, and 9wt%. The measurements revealed that the IFT was reduced from 34.9 mN/m (distilled water/toluene) to a minimum value of 25.3 mN/m for the resinous synthetic oil prepared by dissolution of resin in the toluene with a concentration of 9 wt%. According to these findings, it seems that the resin fraction can act as a natural surfactant in the light of its structure and heteroatoms of sulfur and oxygen existing in the resin structure.
A closer look (Fig. 1) revealed that as the pH value increased, the IFT was reduced due to the acidic nature of the resin fraction interacting with the solution contents which produced in situ surfactant, consequently empowering the surfactant-nature of the dissolved resin fraction in the toluene.
Effect of ionic liquids on interfacial tension
In the next stage of this investigation, the effects of two different ILs from pyridinium and imidazolium families concomitant with the different concentrations of MgCl2 and CaCl2 in the range of 1000–41000 ppm were examined (see Figs. 2, 3, 4, 5). The measurements of this section are divided into two subsets, one of them was performed using distilled water as the aqueous solution containing ILs as the surfactant, and the second set of experiments was performed using two different salts of MgCl2 and CaCl2 with different concentrations concomitant with the dissolved ILs. The depicted results in Fig. 2a–c revealed that increasing the ILs concentrations in the absence and presence of salts leads to a reduction in IFT due to the surfactant nature of the ILs. Besides, the overall observed trend revealed that using [C12py][Cl] leads to a better reduction in IFT compared with the IFT reduction that occurred by [C12mim][Cl] due to the higher acidic nature of the imidazolium compared with the pyridinium for all the examined pH values and resin fractions. However, using [C12mim][Cl] revealed better functionality for IFT reduction in the solution with a pH value of 11 and resin fraction of 9 wt%. In detail, imidazolium cation is an acidic component with pKa = 21–23 which comes from the H2 hydrogen of the imidazolium nucleus while pyridine is often used as an organic base in chemical reactions pKa = 5. In light of this fact, it seems that since the acidic resin fraction is in contact with a basic surfactant neutralization of repulsive forces occurred leading to easier packing of the pyridinium-based ILs in the interface consequently leading to more IFT reduction compared with the imidazolium-based IL.
But in the case of an aqueous solution with a pH value of 11 and synthetic oil with a resin fraction of 9 wt%, the condition was completely different. The reason behind this observed shifting behavior can be attributed to the formation of complexes between the [C12py][Cl] and resin molecules producing overwhelming repulsive forces that move the IL molecules toward the bulk instead of packing them into the interface consequently leading to higher IFT values than the [C12mim][Cl]/synthetic resinous oil system. The other point that can be extracted from the depicted results in Fig. 2 is that for both examined ILs and pH values, a sharp critical micelle concentration (CMC) value of 400 ppm was obtained since most of the IFT reduction occurred if the IL concentration increases from 0 to 400 ppm while further increase in the IL concentration from 400 to 1000 ppm led to a lower reduction in IFT values.
In the second series of experiments, the effects of ILs were examined on the IFT reduction under different pH values and in the presence of MgCl2 and CaCl2 (in the range of 500–50000 ppm) (see Fig. 3). The point is that in the current phase, central composite design (CCD) approach was used as the design of experiment method instead of changing one factor at a time method to reduce the number of required measurements since the number of experiments would be extensive if changing one factor at a time approach was used. In this way, the parameters including IL type (imidazolium-based and pyridinium-based), pH value (3–11), resin fraction (1–9 wt%), and MgCl2 and CaCl2 concentrations (1000–41000) were used as the independent parameters (see Table 1). According to the intervals tabulated in Table 1 and 2, the IFT measurements were performed and used to find the optimum operating condition besides the possible interactions that exist between different parameters.
Packing of NPs in the crude oil/aqueous phase interface (Nowrouzi et al. 2019)
The point is that two different sets of experiments were performed based on the CCD approach for two ILs of [C12mim][Cl] and [C12py][Cl] while the other operating parameters of pH value, resin fractions in toluene, and salinities were the same. With a close look into the results obtained for [C12mim][Cl], one can conclude that the quadratic model can predict the IFT values for different conditions with an acceptable level of accuracy (see Table 3 and 4).
In general, a glance into the results tabulated in Table 3 revealed that the F value for the model is about 45.99 means that there is only a 0.01% chance that a large value for “Model F value” occurred due to the noise. Moreover, the “Prob > F” parameter (for the values lower than 0.05) is an indication which revealed that all of the examined parameters of pH, salt concentrations, IL concentration, and resin fraction are effective parameters their effect must be taken into account. Besides, a closer look into the results revealed that not only the individual parameters have a profound effect on the IFT but also the interactions that existed between the MgCl2/[C12mim] [Cl] and CaCl2/[C12mim][Cl] are dominant among the other interactions. On the other side, if the “Prob > F” values are greater than 0.1 as tabulated in Table 3, it is an indication that those parameters are insignificant in the model.
In the next stage, the error analysis namely the sequential model sum of squares and lack of fit tests was performed to find the most accurate and proper models which directed the conclusions toward the quadratic model (see Table 4). In the last stage, the proposed quadratic model (see Eq. 2 for the fitting parameters of each constant) was analyzed to find the accuracy of this model.
The obtained results revealed that considering the standard deviation and PredR-Squared concomitant with Adj R-Squared which values are about 0.8729 and 0.9484, respectively, one can conclude that the proposed model is accurate enough to be used to predict the IFT values of the mixtures in different intervals in the examined ranges of the parameters. Besides, the Adeq Precisions which is the ratio of signal to noise ratio is an indication of the capability of the proposed model to navigate the designed space. In detail, since the “Adeq Precision” is well above the value of 4 (32.819), the proposed model is well-suited for predicting the IFT value of the mixture under different conditions (see Table 5).
In the next stage of this section, the effect of [C12py][Cl] on the IFT in the presence of different salts under different operating conditions was examined using the CCD approach (Table 2 and 6). The obtained results revealed that similar to the results obtained for the solutions comprised of [C12mim][Cl], operating conditions including pH, CaCl2, and MgCl2 concentrations, and resin fraction introduced a profound effect on the IFT value. The point is that although similar to the results obtained for [C12mim][Cl], the interactions between [C12py][Cl] and CaCl2 and MgCl2 are significant, all the interactions between pH/CaCl2, pH/MgCl2, MgCl2/Resin fraction, CaCl2/Resin fraction, and [C12py][Cl]/resin fraction are significant and undeniable. In this way, it seems that in contrast to the results obtained for [C12mim][Cl], the presence of [C12py][Cl] in the mixture solution has a significant effect on all of the examined parameters. This observed trend can be correlated to the fact that imidazolium cation is an acidic component with pKa = 21–23 which comes from the H2 hydrogen of the imidazolium nucleus while pyridine is often used as an organic base in chemical reactions pKa = 5. In light of this fact, it seems that since the resin fraction has an acidic nature and is contacted with a basic surfactant neutralization of repulsive forces existed between the molecules occurs leading to easier packing of the pyridinium-based ILs in the interface consequently leading to more IFT reduction compared with the imidazolium-based IL.
The analysis of the obtained results through the modeling stage revealed that similar to the results obtained for the solutions including [C12mim][Cl], the best model in the quadratic model can accurately model the IFT values of the mixtures including [C12py][Cl] (see Table 7). Besides, further analysis revealed that not only the “sequential model sum of squares” revealed that the quadratic model is the best, but also the “lack of fit tests” confirmed the capability of the quadratic model (see Table 7).
Moreover, the calculated “Adeq precision” value which is about 47.339 is a good indication for the capability of the proposed model for the IFT prediction since the “Adeq precision” value must be above 4 to indicate the potential of the proposed model for accurate performance (see Table 8).
As a final point regarding the proposed quadratic model, one can use the fitting parameters tabulated in Table 10 to reproduce the measured IFT values for the different operating conditions and solutions prepared by the dissolution of different chemicals (see Eq. 3).
The worth mentioning point is that a glance into the operating parameters and measured IFT values for both data sets determined using the CCD approach revealed that Run#22 and Run#26 led to the lowest IFT values of 2.2 and 0.62 mN/m. In this way, these two solutions were selected and used to find the effect of these two solutions on the wettability alteration considering the initial condition of the rock wettability which was strongly oil-wet with a contact angle of about 125.3°. The measurements revealed that the application of this aqueous solution in the presence of synthetic resinous oil with different concentrations of 1, 3, 5, 7, and 9 wt% led to the reduction in contact angle revealed the movement of the rock wettability toward water-wet conditions (see Table 11).
Effect of optimum formulation on wettability alteration
The measurements revealed that for both examined optimum formulations of [C12mim][Cl] and [C12py][Cl] in contact with the synthetic resinous oil prepared using different resin fractions, as the resin fraction increases from 1 to 9 wt%, a reduction in contact angle from 67.3° to 51.2o for [C12mimi][Cl] and from 62.3° to 42.2° for [C12py][Cl]. A closer look into the tabulated results in Table 9 revealed a better functionality of [C12py][Cl] for changing the wettability of the rock surface toward water-wet conditions. However, an increase in the resin fraction has a moderate effect on the movement of the rock wettability toward strongly water-wet conditions.
Aqueous solution stability at the presence of TiO2-NPs
In the second stage of this investigation, the stability of the aqueous solution containing TiO2-NPs was investigated since precipitation of NPs in the reservoir is an undeniable risk that must be reduced to its minimum level or even eliminated precipitation and pore plugging. In this way, compatibility tests must be performed to find the concentration and pH value leading to the most stable aqueous solution containing TiO2-NPs for a long period and at least one month. In this way, considering the optimum chemical formulation for each IL (led to the lowest IFT values), the compatibility tests were performed by changing the TiO2-NPs concentration between 0 and 200 ppm. All of the prepared solutions revealed long-term stability (more than four weeks) in the presence of ILs regardless of the IL type while the aqueous solutions prepared without ILs experienced precipitation after 5 h of solution preparation. As a consequence of these observations, it seems that the application of IL is a crucial parameter to stabilize the TiO2-NPs for a long period. Respecting these facts, using the optimum formulation along with the TiO2-NPs has the minimum risk of precipitation due to the results obtained during the compatibility test stage.
The point that must be clarified is that the maximum concentration of TiO2-NPs was selected at 200 ppm since as the concentration of TiO2-NPs was increased to values higher than 200 ppm, the transparency of the solution faded which put the operator in trouble to measure the IFT in contact with the dark solution of the synthetic oil.
Effect of TiO2-NPs on the IFT
In the next phase of this investigation, the synergistic effect of TiO2-NPs was investigated by measuring the IFT value between the aqueous solution/synthetic resinous oil by ranging the TiO2-NPs concentration between 0–200 ppm, and the chemical formulations were kept constant as obtained for Run#22 and Run#26. The worth mentioning point is that although the chemical formulations obtained for Run#26 and Run#22 are in contact with specific fractions of resin dissolved in toluene (9 wt% for [C12mim][Cl] and 7 wt% for [C12py][Cl], respectively), the IFT measurements of this section were performed using different resin fractions in the range of 1–9 wt% (see Table 10 and 11).
The measured IFT values revealed that an increase in the TiO2-NPs concentration led to a reduction in the IFT from the maximum value of 2.2 to 0.62 mN/m for [C12mim][Cl] and from 6.8 to 0.84 mN/m for [C12py][Cl] which all of this minimum IFT values was obtained if the TiO2-NPs concentration was increased to 200 ppm. The point is that for all of the TiO2-NPs concentrations and different resin fractions dissolved in toluene, the IFT values were measurable except the solutions were prepared by 100 ppm and 200 ppm of TiO2-NPs and resin fractions of 9 wt% which led to IFT values lower than 0.4 mN/m made it impossible to measure the IFT values for those solutions using pendant drop method (see Table 12). Similar results were obtained for the effect of TiO2-NPs concentration by Nowrouzi et al. (2019) who examined the impact of TiO2-NPs on the IFT reduction using different concentrations of 500 and 1000 ppm in the formation brine and diluted formation brine which were carbonated using CO2 dissolution.
In detail, they reported that the presence of γ-Al2O3-NPs, MgO-NPs, and TiO2-NPs are so effective for IFT reduction, especially γ-Al2O3-NPs. For example, under similar conditions of 1000 ppm of NPs, the salinity of 10-times diluted water (10 times), and under pressure and temperature of 13.79 MPa, and 40 °C, respectively, the IFT values were γ-Al2O3, MgO, and TiO2 are 2.85, 3.31, and 6.43 mN/m, respectively. Although they examined the effect of NPs in the presence of carbonated water, the mechanism which is correlated to the NPs are the same since NPs can produce a monolayer depending on the type and size of NPs which can boost the packing of different molecules and ions at the interface leading to lower IFT values. In brief, based on the theory and mechanism proposed by Li et al. (2013) and Dahle (2013) it is reported that NPs can produce a layer between nanofluid and crude oil in the interface that reduces the IFT between the immersed phases. The functionality of this formed layer is usually considered similar to the layer that the surfactant molecules can produce. The noteworthy point is that although the effect of surfactant concentration on the IFT is mostly in a linear trend, the impact of NPs concentration is not similar to the surfactant molecules (Dahle 2013; Hendraningrat et al. 2013a).
As described in Fig. 3 (Nowrouzi et al. 2019), the formation of nanofilms on the interface of the drop/bulk in the presence of ions moves the NPs toward a more harmonic and tidy pattern in the interface, especially if the number of NPs are less than the ions. As a consequence of this tidiness, the entropy of the whole suspension increases by increasing the freedom for nanoparticles leading to higher pressure on the volume (Chengara et al. 2004). The point is that the NPs form a thin film consisting of the interface which is the consequence of structural disjoining pressure with the source of the Brownian motion and electrostatic repulsion between NPs. In this regard, Aveyard et al. reported that the formation of this nanofilm is highly dependent on the NPs concentration and size, salinity, temperature, and interfacial properties. For example, as the amount NPs concentration increases or the size of NPs reduces in the nanofluid, the film progression increases on the interface. The other possible reason behind the effect of NPs on the IFT is the fact that NPs are not generally amphiphiles but an increase in surface pressure is measured on their adsorption due to a decrease in the IFT caused by the appropriate wettability of the nanoparticles at the interface of oil and water (Binks 2002) [82].
In this way, the ILs with bulky structures can reduce the interfacial tension in the first stage. After that, in light of this reduction in IFT, the NPs easily move toward the interface to form a progressed thin film layer of NPs which is essential to modify the IFT and wettability alteration. Besides, the bulky structure of the ILs provides a proper chance for the NPs to be packed in the IL structure for easier packing and arrangement in the interface. As a consequence of this higher amount of packed NPs in the interfaces, a thinner film of NPs would be formed in the interface leading to lower IFT values and probably better wettability alteration toward the strongly water-wet condition. Moreover, the presence of IL can reduce the repulsive forces between the NPs means a higher amount of NPs can be packed in the interface leading to lower IFT values.
Wettability alteration
In the last stage of this investigation, the effect of optimum formulations was examined on the CA of the thin sections prepared from the carbonate rocks which were aged under the temperature of 80 °C and pressure of 1000 psi for 1 month to restore them to the oil-wet condition (similar to the reservoir condition) (see Table 12 and 13).
A close look into the results tabulated in Table 12 and 13 revealed that increasing the concentration of TiO2-NPs in both aqueous solutions of [C12mim][Cl] and [C12py][Cl] leads to a considerable reduction in the CA which means the strongly water-wet conditions. This observed effect on the CA variation is correlated to the effects of ILs, and TiO2-NPs can synergistically move the rock wettability toward strong water-wet conditions. In detail, the penetration of surfactant molecules into the oil film existing on the rock surface can change the rock wettability to more water-wet status. As a consequence of this penetration, the surfactant molecules stick to the rock surface rendering the rock surface toward more water-wet conditions. In detail, the presence of surfactants in the aqueous solution can manipulate the key interactions responsible for the wettability alteration such as the electrostatic force, the hydrophobic force, and the attractive force between the surfactant and polar components of the crude oil and rock morphology. In general, the wettability alteration of an oil-wet carbonate rock surface depends upon the type of surfactant, oil composition, the concentration of surfactant, and brine salinity. The other point which can manipulate the efficiency of the surfactant for wettability alteration is the presence of salts in the aqueous solutions. In detail, salts can also accelerate the diffusion of surface-active constituents from the bulk solution to the interface and, thus, enhance the adsorption of the surfactant at the interface, resulting in the decrease of the rock surface from intermediate-wet to water-wet increasing the oil recovery rate.
Regarding the effect of NPs on the wettability alteration, a similar trend was observed by Hendraningrat and Torsæter (2014) who examined the effect of silica-based nanoparticles on the IFT reduction, wettability alteration, and tertiary oil recovery efficiency. They reported that using silica-based NPs can change the wettability toward water-wet conditions. In detail, they reported the wettability alteration in the presence of silica NPs due to changes in the solid/fluid interface caused by the adsorption of hydrophilic NPs. They also reported that the degree of water-wetness increased in both water- and oil-wet systems as shown by the smaller contact angle of the aqueous phase. They observed that once the hydrophilic NPs are introduced into the synthetic seawater (SSW), the NPs reduce the contact angle from 39° to 26° (a 33% reduction), which indicates that NPs have rendered the quartz plate toward a more SWW system. In an oil-wet system, the presence of hydrophilic NPs in an SSW system tends to change the quartz plate to a weakly oil-wet system from 131° to 112° (a 15% reduction). Hence, the surface quartz wettability was modified and will favor the aqueous phase. Also, Morrow reported considerable wettability changes for quartz surfaces were due to the adsorption of a monolayer of polar molecules. However, the use of a quartz plate has limitations in using a rock surface as a single mineral (quartz), as reported by Morrow (1990).
Moreover, Ershadi et al. (2015) utilized hybrid carbon/silica nanotubes to change the rock wettability of surfaces toward a water-wet state. It was reported by Ershadi et al. (2015) that the value for the CA increases, and that explains the capability of this hybrid combination of nanoparticles to render the rock surface more water-wet while their investigation revealed the dual effects of the used NPs on the modification of both wetness change and IFT. The reduction of interfacial tension, IFT is the most effective mechanism that might enhance oil production. According to the findings, it is possible to categorize the possible mechanisms regarding NPs into three different classes, and one of the most important ones besides disjoining pressure and density difference is changing wetness. The application of NPs can directly produce a gradient between reservoir fluids and injecting fluid by manipulating the density of the fluid can consequently affect the movement of oil film on the surface of rocks and replace it with water film which means changing the oil-wet condition (Al-Anssari et al. 2016; Maghzi et al. 2011; Wang et al. 2005).
Besides, Wasan et al. (2011) performed a visual-video microscopy-based investigation to examine solid surface modification with the assistance of immiscible liquid spreading on a solid surface occurring to Brownian motion. Their findings revealed that the NPs arrangement leads to the formation of a wedge film between the oil and solid substrates (see Figs. 4 and 5. At this point, a pressure gradient appears between the thin layer and the bulk of fluid and is boosted as the NPs concentration enhances. As a consequence of this pressure gradient, it would be possible to manipulate by affecting the film tension toward the vertex of the wedge, consequently influencing the surface wettability (Kondiparty et al. 2011; Wasan et al. 2011).
Ordering of nanoparticle in the wedge-film resulting in structural disjoining pressure gradient at the wedge vertex (Salehi et al. 2010)
Besides the other NPs, Aghajanzadeh et al. (2019) have investigated the effect of silica-nanoparticles on the changing wetness of rock surfaces. They have reported that according to the obtained results the concentrations of NPs enhance the changing wetness was shifted from oil-wet to water-wet. Moreover, the results of spontaneous imbibition examinations revealed an increase of about 15% higher oil recovery using nanofluid compared with the case only formation brine was utilized. Finally, they examined the effect of NPs presence in the injected solution during the core flooding experiments and found that not only the relative permeability curve was modified and moved toward the right side of the curve also the water relative permeability was reduced to 0.23 mD from its initial value of 0.43 mD at the residual oil saturation due to wetness change toward water wet status from strongly oil-wet (Aghajanzadeh et al. 2019).
On the other hand, an innovative application of nanoparticles with anionic surfactants was performed by Ershadi et al. (2015) illustrating the possible effect of surfactant existence that might reduce the IFT values. As well as maybe, accelerate the penetration of NPs to enhance their impact on the rock surface wettability, moreover, the reduced IFT and the wetness change strengthen one another, and that definitely will enhance the oil production by producing more trapped oil from the reservoirs (Resasco et al. 2015). Furthermore, Karimi et al. (2012) utilized Zirconium oxide (ZrO2) to alter the rock wetness surfaces. In addition, it was found that the adsorption of these NPs might modify the surface toward neutral wet, as well as it was reported that measuring the air/water CA values demonstrated the change in wettability from oil-wet to neutral wetting states. In addition, it was found the energy-dispersive X-ray, EDX, and the analysis of the samples aged in a solution containing NPs of ZrO2 revealed that the surfaces are composed of Ca and Zr as constituent materials, which means zirconium, was adsorbed onto the surface [8]. One of the other innovative investigations is related to the production of a new family of NPs, e.g., Fe3O4 NPs coated nanoparticle Ethylenediaminetetraacetic (EDTA) as a hydrophilic polymer or Sodium Lauryl Sulfate (SLS) as an anionic surfactant by the dip-coating method recently proposed by Shalbafan et al (2019). In this method, a nanoparticle can effectively change the wettability of rock surfaces from oil-wet to water-wet conditions by activating the disjointing pressure mechanism.
The last point is that the experiments performed by Hendraningrat and Torsæter (Hendraningrat and Torsæter 2014) revealed that using the optimal nano-EOR condition reduced the residual oil saturation and increased the displacement efficiency in all wettability systems. They also reported that increasing the temperature can enhance the efficiency of the displacement mechanism. Finally, they performed an extended post-flush nano-flooding revealing the possibility of producing more oil from the core plugs with maximum incremental oil recovered up to 4.9% of OOIP. According to the findings of Hendraningrat and Torsæter (Hendraningrat and Torsæter 2014) and those obtained in the current investigation, it seems that combining the SiO2-NPs with ILs as the chemical surfactant and stabilizer can provide a more efficient chemical formulation for oil recovery purposes.
Conclusions
The application of 1-dodecyl 3-methyl pyridinium chloride ([C12py][Cl]) and 1-dodecyl 3-methyl imidazolium chloride ([C12mim][Cl]) as novel surfactants (0–1000 pm) in the absence and presence of titanium oxide nanoparticles (TiO2-NPs) (0–200 ppm) besides pH (3–11) and divalent salts namely magnesium chloride and calcium chloride in the range of 1000–41000 ppm are investigated. Besides, instead of using crude oil comprised of many components, synthetic resinous oil was used for interfacial tension (IFT) and wettability alteration measurements using the central composite design (CCD) and changing one factor at a time to reduce the required number of experiments. Respecting this approach, the following results were obtained:
-
The presence of resin acts as the natural surfactant reducing the interfacial tension to minimum value of 25.3 mN/m in the absence of different chemicals.
-
In the absence of salts, pH, and TiO2-NPs, [C12py][Cl] leading to lower IFT values than [C12mim][Cl] due to the acidic nature of the resin fraction and basic nature of [C12py][Cl] leading to the positive interaction in the interface which can effectively reduce the IFT.
-
The minimum IFT value was obtained for pH = 7, MgCl2 concentration = 21,000 ppm, CaCl2 concentration = 21,000 ppm, resin fraction of 9 wt% and 500 ppm of [C12mim][Cl] concentration.
-
The positive interaction that existed between the different operating parameters put the [C12mim][Cl] in a better situation for IFT reduction than the [C12py][Cl] although in the absence of different chemicals, [C12py][Cl] leading to better functionality.
-
Combining the TiO2-NPs with the optimum chemical formulations for [C12mim][Cl] and [C12py][Cl] shifted the wettability toward strongly water-wet condition.
-
The CA values are reduced to values of 33.2° and 27.3° for [C12mim][Cl] and [C12py][Cl] with TiO2 concentration of 200 ppm and resin fraction of 9wt%.
-
The IFT measurements revealed that the presence of TiO2-NPs not only moved the wettability toward strongly water-wet condition, but also the IFT of both chemical formulations for ILs experienced a reduction to minimum values of 0.55 mN/m and 0.84 mN/m for [C12mim][Cl] and [C12py][Cl].
As the last point, although the obtained results revealed the efficiency of the obtained optimum formulation on the IFT reduction and wettability alteration toward desired conditions, the practical applications of these optimum formulations must be examined using other experiments. For example, it seems highly applicable if the obtained optimum formulations are used in the core flooding experiments and adsorption tests analysis which can be the next phase of similar investigations currently performed. These two experiments and tests are necessary since they can provide better insight regarding the required amount of chemicals used in the optimum formulations (cost evaluation) and the possible tertiary oil recovery in the field scale (feasibility and capability). Moreover, the impact of these formulations must be examined in the presence of reservoir heterogeneities since the rock interactions with fluids have a significant effect on the efficiency of the optimum formulation.
Abbreviations
- D :
-
Equatorial diameter (m)
- d :
-
Diameter at the distance D from the top of the drop (m)
- g :
-
Acceleration of gravity (m s−2)
- H :
-
Shape-dependent parameter (m)
- L :
-
Drop length (m)
- R and R m :
-
Radius of the drop at equator (m)
- R o :
-
Radius of the drop at edge (m)
- γ :
-
Interfacial tension (mN/m)
- Δ :
-
Difference between two parameters
- ρ :
-
Density (kg m−3)
- ω :
-
Rotational speed (rad/s)
- Al2O3 :
-
Aluminum oxide
- ANOVA:
-
Analysis of variance
- APIo :
-
API gravity
- ASO:
-
Asphaltenic synthetic oil
- ASTM:
-
American Society for Testing and Materials
- CA:
-
Contact angle
- CaCl2:
-
Calcium chloride
- CCD:
-
Central composite design
- CMC:
-
Critical micelle concentration
- EDTA:
-
Ethylenediaminetetraacetic
- EOR:
-
Enhanced oil recovery
- Fe2O3 :
-
Iron oxide
- Fe3O4 :
-
Iron(II,III) oxide
- GC:
-
Gas chromatography
- IFT:
-
Interfacial tension
- ILs:
-
Ionic liquids
- MgCl2:
-
Magnesium chloride
- NPs:
-
Nanoparticle
- OOIP:
-
Original oil in place
- pH:
-
Potential of hydrogen/power of hydrogen
- ppm:
-
Part per million
- PSNP:
-
Polysilicon NPs
- RSO:
-
Resinous synthetic oil
- SiO2:
-
Silicon oxide
- SiO2-NPs:
-
Silicon oxide nanoparticles
- SLS:
-
Sodium lauryl sulfate
- TiO2:
-
Titanium oxide
- WNW–ESE:
-
West-northwest–east-southeast
- NE–SW:
-
Northwest–southwest
- wt%:
-
Percentage by weight
- ZrO2:
-
Zirconium dioxide
- [C12mim][Cl]:
-
1-Dodecyl-3-methyl imidazolium chloride
- [C12py][Cl]:
-
1-Dodecyl-3-methyl pyridinium chloride
References
Abhishek R, Kumar GS, Sapru R (2015) Wettability alteration in carbonate reservoirs using nanofluids. Pet Sci Technol 33(7):794–801
Aghajanzadeh MR, Ahmadi P, Sharifi M, Riazi M (2019) Wettability modification of oil-wet carbonate reservoirs using silica-based nanofluid: an experimental approach. J Petrol Sci Eng 178:700–710
Al-Anssari S, Barifcani A, Wang S, Maxim L, Iglauer S (2016) Wettability alteration of oil-wet carbonate by silica nanofluid. J Colloid Interface Sci 461:435–442
Andersen SI (1994) Effect of precipitation temperature on the composition of n-heptane asphaltenes. Fuel Sci Technol Int 12(1):51–74
Anganaei H, Pourabdollah K, Rostami A (2014) Experimental improvement of nano-enhanced oil recovery using nano-emulsions. Arab J Sci Eng 39(8):6453–6461
Aveyard R, Binks BP, Clint JH (2003) Emulsions stabilised solely by colloidal particles. Adv Coll Interface Sci 100:503–546
Bera A, Belhaj H (2016) Application of nanotechnology by means of nanoparticles and nanodispersions in oil recovery—a comprehensive review. J Nat Gas Sci Eng 34:1284–1309
Berberian M, King G (1981) Towards a paleogeography and tectonic evolution of Iran. Can J Earth Sci 18(2):210–265
Beydoun ZR (1991) Arabian plate hydrocarbon geology and potential—a plate tectonic approach
Binks BP (2002) Particles as surfactants—similarities and differences. Curr Opin Colloid Interface Sci 7(1–2):21–41
Bobbo S et al (2012) Viscosity of water based SWCNH and TiO2 nanofluids. Exp Thermal Fluid Sci 36:65–71
Bosold A, Schwarzhans W, Julapour A, Ashrafzadeh A, Ehsani S (2005) The structural geology of the High Central Zagros revisited (Iran). Pet Geosci 11(3):225–238
Chehrazi A, Rahimpour-Bonab H, Rezaee M (2013a) Seismic data conditioning and neural network-based attribute selection for enhanced fault detection
Chehrazi A, Rahimpour-Bonab H, Rezaee M (2013b) Seismic data conditioning and neural network-based attribute selection for enhanced fault detection. Pet Geosci 19(2):169–183
Chen L et al (2014) Inexpensive ionic liquids:[HSO 4]−-based solvent production at bulk scale. Green Chem 16(6):3098–3106
Chengara A, Nikolov AD, Wasan DT, Trokhymchuk A, Henderson D (2004) Spreading of nanofluids driven by the structural disjoining pressure gradient. J Colloid Interface Sci 280(1):192–201
Cheraghian G (2015) Effects of nanoparticles on wettability: a review on applications of nanotechnology in the enhanced oil recovery
Cheraghian G (2016) Effects of titanium dioxide nanoparticles on the efficiency of surfactant flooding of heavy oil in a glass micromodel. Pet Sci Technol 34(3):260–267
Cheraghian G, Hendraningrat L (2016) A review on applications of nanotechnology in the enhanced oil recovery part B: effects of nanoparticles on flooding. Int Nano Lett 6(1):1–10
Dahle G (2013) The effect of nanoparticles on oil/water interfacial tension. Project thesis, NTNU
de Castro Dantas TN, de Souza TTC, Neto AAD, de Alencar Moura MCP, de Barros Neto EL (2017) Experimental study of nanofluids applied in EOR processes. J Surfact Deterg 20(5):1095–1104
Demirbas A (2016) Deposition and flocculation of asphaltenes from crude oils. Pet Sci Technol 34(1):6–11
Demirbas A, Alidrisi H, Balubaid M (2015) API gravity, sulfur content, and desulfurization of crude oil. Pet Sci Technol 33(1):93–101
Demirbas A, Taylan O (2015) Recovery of gasoline-range hydrocarbons from petroleum basic plastic wastes. Pet Sci Technol 33(23–24):1883–1889
Dharaskar Swapnil A (2012) Ionic liquids (a review): the green solvents for petroleum and hydrocarbon industries. Res J Chem Sci ISSN 2231:606
Domańska U (2005) Solubilities and thermophysical properties of ionic liquids. Pure Appl Chem 77(3):543–557
Ehtesabi H, Ahadian MM, Taghikhani V (2015) Enhanced heavy oil recovery using TiO2 nanoparticles: investigation of deposition during transport in core plug. Energy Fuels 29(1):1–8
El-Diasty AI, Ragab AM (2013) Applications of nanotechnology in the oil & gas industry: Latest trends worldwide & future challenges in Egypt, North Africa Technical Conference and Exhibition. OnePetro
Ershadi M, Alaei M, Rashidi A, Ramazani A, Khosravani S (2015) Carbonate and sandstone reservoirs wettability improvement without using surfactants for Chemical Enhanced Oil Recovery (C-EOR). Fuel 153:408–415
Fard IA, Braathen A, Mokhtari M, Alavi SA (2006) Interaction of the Zagros Fold-Thrust Belt and the Arabian-type, deep-seated folds in the Abadan Plain and the Dezful Embayment. SW Iran Pet Geosci 12(4):347–362
Ghazban F (2007) Petroleum geology of the Persian Gulf. Joint publication Tehran University Press and National Iranian Oil Company, Tehran
Hashemi R, Nassar NN, Pereira Almao P (2013) Enhanced heavy oil recovery by in situ prepared ultradispersed multimetallic nanoparticles: a study of hot fluid flooding for Athabasca bitumen recovery. Energy Fuels 27(4):2194–2201
Hendraningrat L, Li S, Torsæter O (2013a) A coreflood investigation of nanofluid enhanced oil recovery. J Petrol Sci Eng 111:128–138
Hendraningrat L, Li S, Torsæter O (2013b) Effect of some parameters influencing enhanced oil recovery process using silica nanoparticles: an experimental investigation, SPE Reservoir Characterization and Simulation Conference and Exhibition. OnePetro. SPE-165955-MS
Hendraningrat L, Li S, Torsaeter O (2013c) A coreflood investigation of nanofluid enhanced oil recovery in low-medium permeability Berea sandstone, SPE International Symposium on Oilfield Chemistry. OnePetro. https://doi.org/10.2118/164106-MS
Hendraningrat L, Li S, Torsaeter O (2013d) Enhancing oil recovery of low-permeability Berea sandstone through optimized nanofluids concentration, SPE enhanced oil recovery conference. OnePetro. https://doi.org/10.2118/165283-MS
Hendraningrat L, Torsæter O (2014) Effects of the initial rock wettability on silica-based nanofluid-enhanced oil recovery processes at reservoir temperatures. Energy Fuels 28(10):6228–6241
Hezave AZ, Dorostkar S, Ayatollahi S, Nabipour M, Hemmateenejad B (2013a) Dynamic interfacial tension behavior between heavy crude oil and ionic liquid solution (1-dodecyl-3-methylimidazolium chloride ([C12mim][Cl]+ distilled or saline water/heavy crude oil)) as a new surfactant. J Mol Liq 187:83–89
Hezave AZ, Dorostkar S, Ayatollahi S, Nabipour M, Hemmateenejad B (2013b) Investigating the effect of ionic liquid (1-dodecyl-3-methylimidazolium chloride ([C12mim][Cl])) on the water/oil interfacial tension as a novel surfactant. Colloids Surf A 421:63–71
James G, Wynd J (1965) Stratigraphic nomenclature of Iranian oil consortium agreement area. AAPG Bull 49(12):2182–2245
José-Alberto M-H, Jorge A (2011) Current knowledge and potential applications of ionic liquids in the petroleum industry. Ionic liquids: applications and perspectives
Ju B, Fan T, Ma M (2006) Enhanced oil recovery by flooding with hydrophilic nanoparticles. China Particuol 4(1):41–46
Karimi A et al (2012) Wettability alteration in carbonates using zirconium oxide nanofluids: EOR implications. Energy Fuels 26(2):1028–1036
Kazemzadeh Y, Eshraghi SE, Sourani S, Reyhani M (2015) An interface-analyzing technique to evaluate the heavy oil swelling in presence of nickel oxide nanoparticles. J Mol Liq 211:553–559
Khalil M, Jan BM, Tong CW, Berawi MA (2017) Advanced nanomaterials in oil and gas industry: design, application and challenges. Appl Energy 191:287–310
Khupse ND, Kumar A (2010) Ionic liquids: new materials with wide applications
Kondiparty K, Nikolov A, Wu S, Wasan D (2011) Wetting and spreading of nanofluids on solid surfaces driven by the structural disjoining pressure: statics analysis and experiments. Langmuir 27(7):3324–3335
Lashkarbolooki M, Ayatollahi S, Riazi M (2014) Effect of salinity, resin, and asphaltene on the surface properties of acidic crude oil/smart water/rock system. Energy Fuels 28(11):6820–6829
Lashkarbolooki M, Riazi M, Ayatollahi S, Hezave AZ (2016) Synergy effects of ions, resin, and asphaltene on interfacial tension of acidic crude oil and low–high salinity brines. Fuel 165:75–85
Lee Y-S, Kim D-W (2013) Cycling performance of lithium polymer cells assembled by in situ polymerization of a non-flammable ionic liquid monomer. Electrochim Acta 106:460–464
Li S, Hendraningrat L, Torsaeter O (2013) Improved oil recovery by hydrophilic silica nanoparticles suspension: 2 phase flow experimental studies, IPTC 2013: International Petroleum Technology Conference. European Association of Geoscientists & Engineers, pp cp-350–00212
Li W et al (2021) Solvent manipulation of the pre-reduction metal–ligand complex and particle-ligand binding for controlled synthesis of Pd nanoparticles. Nanoscale 13(1):206–217
Maghzi A, Mohebbi A, Kharrat R, Ghazanfari MH (2011) Pore-scale monitoring of wettability alteration by silica nanoparticles during polymer flooding to heavy oil in a five-spot glass micromodel. Transp Porous Media 87(3):653–664
Martins MA et al (2014) Impact of the cation symmetry on the mutual solubilities between water and imidazolium-based ionic liquids. Fluid Phase Equilib 375:161–167
McElfresh P, Olguin C, Ector D (2012) The application of nanoparticle dispersions to remove paraffin and polymer filter cake damage. SPE International Symposium and Exhibition on Formation Damage Control. OnePetro. SPE-151848-MS
Mohammadi M, Dadvar M, Dabir B (2017) TiO2/SiO2 nanofluids as novel inhibitors for the stability of asphaltene particles in crude oil: mechanistic understanding, screening, modeling, and optimization. J Mol Liq 238:326–340
Moradi B, Pourafshary P, Jalali F, Mohammadi M, Emadi M (2015) Experimental study of water-based nanofluid alternating gas injection as a novel enhanced oil-recovery method in oil-wet carbonate reservoirs. J Nat Gas Sci Eng 27:64–73
Morrow NR (1990) Wettability and its effect on oil recovery. J Petrol Technol 42(12):1476–1484
Mozaffari S (2015) Rheology of Bitumen at the onset of asphaltene aggregation and its effects on the stability of water-in-oil emulsion
Mozaffari S, Ghasemi H, Tchoukov P, Czarnecki J, Nazemifard N (2021) Lab-on-a-chip systems in asphaltene characterization: a review of recent advances. Energy Fuels 35(11):9080–9101
Mozaffari S, Tchoukov P, Atias J, Czarnecki J, Nazemifard N (2015) Effect of asphaltene aggregation on rheological properties of diluted athabasca bitumen. Energy Fuels 29(9):5595–5599
Muhammad I et al (2013) SARA separation and determination of concentration levels of some heavy metals in organic fractions of Nigerian crude oil. Chem Mater Res 3(4):7–14
Negin C, Ali S, Xie Q (2016) Application of nanotechnology for enhancing oil recovery—a review. Petroleum 2(4):324–333
Nowrouzi I, Manshad AK, Mohammadi AH (2019) Effects of TiO2, MgO, and γ-Al2O3 nano-particles in carbonated water on water-oil interfacial tension (IFT) reduction in chemical enhanced oil recovery (CEOR) process. J Mol Liq 292:111348
Onyekonwu MO, Ogolo NA (2010) Investigating the use of nanoparticles in enhancing oil recovery, Nigeria Annual international conference and exhibition. OnePetro
Peng X-M, Hu Y-F, Jin C-W (2011) Solubilities of imidazolium-based ionic liquids in aqueous salt solutions at 298.15 K. J Chem Thermodyn 43(8):1174–1177
Resasco DE et al (2015) Method and foam composition for recovering hydrocarbons from a subterranean reservoir. Google Patents
Rodríguez-Palmeiro I, Rodríguez-Escontrela I, Rodríguez O, Arce A, Soto A (2015) Characterization and interfacial properties of the surfactant ionic liquid 1-dodecyl-3-methyl imidazolium acetate for enhanced oil recovery. RSC Adv 5(47):37392–37398
Rodriguez Pin E, Roberts M, Yu H, Huh C, Bryant SL (2009) Enhanced migration of surface-treated nanoparticles in sedimentary rocks. Society of Petroleum Engineers, SPE annual technical conference and exhibition. https://doi.org/10.2118/124418-MS
Saien J, Gorji AM (2017) Simultaneous adsorption of CTAB surfactant and magnetite nanoparticles on the interfacial tension of n-hexane–water. J Mol Liq 242:1027–1034
Salehi M, Johnson SJ, Liang J-T (2010) Enhanced wettability alteration by surfactants with multiple hydrophilic moieties. J Surfactants Deterg 13(3):243–246
Seid Mohammadi M, Moghadasi J, Naseri S (2014) An experimental investigation of wettability alteration in carbonate reservoir using γ-Al2O3 nanoparticles. Iran J Oil Gas Sci Technol 3(2):18–26
Setudehnia A (1978) The mesozoic sequence in south-west Iran and adjacent areas. J Pet Geol 1(1):3–42
Shalbafan M, Esmaeilzadeh F, Safaei A (2019) Experimental investigation of wettability alteration and oil recovery enhance in carbonate reservoirs using iron oxide nanoparticles coated with EDTA or SLS. J Petrol Sci Eng 180:559–568
Sheng JJ (2010) Modern chemical enhanced oil recovery: theory and practice. Gulf Professional Publishing
Smit B et al (1991) Structure of a water/oil interface in the presence of micelles: a computer simulation study. J Phys Chem 95(16):6361–6368
Soorghali F, Zolghadr A, Ayatollahi S (2014) Effect of resins on asphaltene deposition and the changes of surface properties at different pressures: a microstructure study. Energy Fuels 28(4):2415–2421
Stauffer CE (1965) The measurement of surface tension by the pendant drop technique. J Phys Chem 69(6):1933–1938
Suleimanov BA, Ismailov F, Veliyev E (2011) Nanofluid for enhanced oil recovery. J Petrol Sci Eng 78(2):431–437
Taborda EA, Franco CA, Lopera SH, Alvarado V, Cortés FB (2016) Effect of nanoparticles/nanofluids on the rheology of heavy crude oil and its mobility on porous media at reservoir conditions. Fuel 184:222–232
Taborda EA, Franco CA, Ruiz MA, Alvarado V, Cortes FB (2017) Experimental and theoretical study of viscosity reduction in heavy crude oils by addition of nanoparticles. Energy Fuels 31(2):1329–1338
Tarek M (2015) Investigating nano-fluid mixture effects to enhance oil recovery. SPE Annual Technical Conference and Exhibition. OnePetro. https://doi.org/10.2118/178739-STU
Torsater O, Engeset B, Hendraningrat L, Suwarno S (2012) Improved oil recovery by nanofluids flooding: an experimental study, SPE Kuwait international petroleum conference and exhibition. Society of Petroleum Engineers. SPE-163335-MS
Yarranton HW, Alboudwarej H, Jakher R (2000) Investigation of asphaltene association with vapor pressure osmometry and interfacial tension measurements. Ind Eng Chem Res 39(2000):2916–2924
Wang D, Duan H, Möhwald H (2005) The water/oil interface: the emerging horizon for self-assembly of nanoparticles. Soft Matter 1(6):412–416
Wasan D, Nikolov A, Kondiparty K (2011) The wetting and spreading of nanofluids on solids: role of the structural disjoining pressure. Curr Opin Colloid Interface Sci 16(4):344–349
Wei L, Zhu J-H, Qi J-H (2007) Application of nano-nickel catalyst in the viscosity reduction of Liaohe extra-heavy oil by aqua-thermolysis. J Fuel Chem Technol 35(2):176–180
Wu J, Prausnitz JM, Firoozabadi A (1998) Molecular-thermodynamic framework for asphaltene-oil equilibria. AIChE J 44(5):1188–1199
Yang Z et al (2014) Interfacial tension of CO2 and organic liquid under high pressure and temperature. Chin J Chem Eng 22(11–12):1302–1306
Zaid HM, Yahya N, Latiff NRA (2013) The effect of nanoparticles crystallite size on the recovery efficiency in dielectric nanofluid flooding. J Nano Res Trans Tech Publ 1:103–108
Zamani A, Maini B, Pereira-Almao P (2012) Flow of nanodispersed catalyst particles through porous media: effect of permeability and temperature. Can J Chem Eng 90(2):304–314
Acknowledgements
Authors thank Islamic Azad University, Dashtestan branch, for their help and financial support in this research.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pazhoohan, M., Hezave, A.Z. Interactions between chloride-based salts (CaCl2 and MgCl2), ionic liquids, pH, and titanium oxide nanoparticles under low and high salinity conditions, and synthetic resinous crude oil: dorud oilfield. J Petrol Explor Prod Technol (2024). https://doi.org/10.1007/s13202-024-01759-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13202-024-01759-x