Abstract
Surface and groundwater are the most common sources of water in Nigeria's rural communities, which are used for a variety of purposes ranging from farming to industrial processes and other domestic household activities including drinking. Water that contains heavy metals in excess of the maximum permitted levels poses a risk to human health. This study aims to evaluate the levels of heavy metals in surface and groundwater in Ifite Ogwari, a rural community in Anambra State, Southeast Nigeria, as well as their ecological indices and human health risks assessment. The concentration levels of Ni, Cr, Cd, Pb, Zn, Fe, Mn, and Cu were determined in fifteen water samples from the major water sources in the study area, viz., streams, river, and hand-dug wells. The water samples were collected using precleaned 500 cm3 glass bottles and were analyzed using Atomic Absorption Spectroscopy (AAS) technique. The results showed that four metals (Cr, Cu, Mn, and Pb) out of the eight heavy metals were not detected in all the samples. The concentration levels of total Ni had a range of 0.029–0.11 mg/L with highest concentration occurring at Isiachala stream, Onowulugbe well, and Omambala river (0.11 mg/L). The Cd levels in the water samples had a range of 0.001–0.036 mg/L, with Isiachala and Iyiutu having the highest values (0.036 mg/L). The concentration of Fe ranged from 0.01 to 0.047 mg/L. Mn was detected at a concentration level of 0.003 mg/L in Iyiutu stream only. The Pearson correlation deduced a strong correlation (> 0.75) and a medium correlation (0.50–0.75) for sample locations and analytes, while three factors (principal component analysis) were produced, which indicates the influence of anthropogenic release rather than natural release. Ecological indices showed the impact of multi-elemental matrices on the ecology, while health risk assessments showed that there was no adverse cancer risk or non-cancer risk across respondents (adults and children). The obtained results showed that anthropogenic release has an extensive mobility influence on the natural level of metals in surface and ground water in Ifite Ogwari, and so proper treatment is advocated. This study has shown that the water sources from Ifite Ogwari pose no adverse health risk to the residents. Consequently, additional research on Ifite Ogwari water is needed to characterize “forever chemicals,” per- and polyfluoroalkyl substances (PFAS) which are ubiquitous, cancerous and have been linked to reproductive and immune system harm, and suggest routes for remediation.
Similar content being viewed by others
Introduction
Water is a basic human amenity and, as such, is extremely important for survival. It is used for a variety of applications, including residential functions, agricultural output, and industrial activities. Despite its importance for life, water is inadequately managed in many regions of the world (Fakayode 2005). Water is a good solvent, and as a result, it dissolves and contains mineral components and other substances that it leaches out when it comes into contact with them. Water contamination in a given location is always proportional to the level of contamination in the surrounding environment (Khan 2011). Rainwater gathers pollutants from the atmosphere as it drips down. As a result, pollutants from surface run-off, sewage discharges, and industrial effluents gather in rivers and streams as they move. Rivers and streams are therefore key sources and conduits for anthropogenic metal mobility and transportation (Stark et al. 2001).
More than a billion people throughout the world lack access to safe drinking water, with more than 300 million of them residing in rural parts of sub-Saharan Africa (Bhatia 2009). Ifite Ogwari, a rural community in Anambra State, Southeastern Nigeria, is characterized by a scarcity of potable water. Because of this, residents must rely on streams, natural ponds/lakes, shallow hand-dug wells, and rainwater collection to provide their whole water demands. It is well known that water resources in rural regions of Nigeria are prone to contamination, either as a result of residents' poor hygiene or as a result of agricultural and local industrial activities (Bolawa et al. 2014). The threat of contaminated drinking water to one’s health is tremendous as many infectious diseases, for example, are spread through water contamination. Drinking contaminated water causes the deaths of five million children each year and sickens one-sixth of the world's population (Essien and Bassey 2012). Heavy metal contamination of surface and groundwater resources is increasingly becoming a major global environmental problem due to their refractory characteristics and bioaccumulation, as evidenced by numerous previous studies such as Adefemi and Awokunmi (2009), Essien and Bassey (2012), Afiukwa et al. (2010), Mudgal et al. (2010), Nkuma (2000). Due to the health dangers associated with their presence, heavy metal pollution of water resources has recently become a focus of public attention (Mitra et al. 2022; Sarker et al. 2022; Balali-Mood et al. 2021). Agricultural activities, houses, local markets, abattoirs, and traditional businesses like blacksmithing, foundries, and metal and crude oil odors have all been identified as substantial anthropogenic sources of heavy metals in the rural aquatic environment (Mudgal et al. 2010). All of these activities degrade the quality of water sources by introducing dangerous toxic metals into them.
Heavy metals form a collection of contaminants that have been discovered as posing a major threat to aquatic habitats and humans, even at trace levels, and are known for their toxicity and environmental durability (Masindi and Muedi 2018). It is widespread knowledge in Nigeria that the bulk of water sources available to locals are contaminated by heavy metals (Adefemi and Awokunmi 2009). As a result, around 60% of Southeastern Nigerians who live in rural areas consume water that is contaminated with germs, heavy metals, and other contaminants that can cause a variety of ailments. Heavy metal contamination and its harmful effects on living species in the aquatic environment and on humans have been studied extensively all over the world and reported by many scholars (Nkuma 2000; Sada and Odemerho 1988; Okafor et al. 2021).
Some hazardous metals are naturally needed for healthy functioning at minute amounts and are referred to as trace elements (iron, copper, manganese, and zinc). These elements are widely distributed in water, beverages, soil, foodstuffs, fruits, and vegetables (Okafor et al. 2016; 2020; 2021a; Nduka et al. 2006; 2008; Orakwue et al. 2021), and are commonly discharged through mining and industrial wastes, automotive emissions, lead-acid batteries, fertilizers, paints, and treated woods (Wuana and Okieimen 2011). Because of their non-biodegradability and lengthy residence period in the environment, hazardous metals like Pb, Cr, and Cd are commonly referred to as “chemical time bombs” (Stiglian et al. 1991). These metals have a direct impact on public health because they are quickly absorbed into the body by oral consumption, skin contact, and/or inhalation (Abrahams 2002). The potential harmful metals’ estimated daily intake rate from multiple pathways (food, soil, water, and air) can be established through ingestion, inhalation, and skin contact (Nadal et al. 2005). The hazard quotient (HQ) developed by the United States’ Environmental Protection Agency (US EPA 2000a; b) has long been used to quantify the possible health risk associated with long-term exposure to hazardous metals in various media. Although these potentially harmful metals occur naturally in the earth's crust, uncontrolled application of agrochemicals, refining, smelting, and burning of fossil fuel and sewage sludge tend to enrich agricultural soil and subsequently water bodies (Gimeno-Garcia et al. 1996; Omokpariola 2021).
Accurate and timely information on water quality is required to develop solid public policy and efficiently conduct the water quality improvement program. The presence of dead vegetation, heavy metal leachates from solid waste dumps, household and industrial sewage, and surface runoff from agricultural farms necessitates research into the heavy metal content of these bodies as drinking water sources. Pollution of these bodies of water first affects the chemical quality of the water, then gradually destroys the community, causing the delicate food web to be disrupted. Because of the ability of water to spread diseases across a wide population, compliance with the heavy metal standard is of particular significance. Although regulations differ from place to place, the goal is to keep the risk of waterborne diseases to a bare minimum while still being pleasant to drink, which means it must be healthy and free of contaminants. As a result, ensuring high-quality drinking water is a critical component of public health, environmental conservation, and long-term growth.
Although Ifite Ogwari is a rural village with little or no industrial activity, it is home to a large number of roadside mechanics, welders, and other artisans. The village is crossed by a major federal route and a state road, which both connect Anambra State to Adani Town in Enugu State (Ihedioha et al. 2021), and within the community, there are a few modest asphalted streets. The use of agrochemicals, indiscriminate dumping of metal-containing wastes, vehicular emissions, and sewage water irrigation are all on the rise in Ifite Ogwari. As a result, it is critical to keep track of the water sources used by locals in Ifite Ogwari, in particular, and Nigeria as a whole. Consequently, Okafor et al. (2022a) reported polycyclic aromatic hydrocarbons (PAHs) contamination of water sources in Ifite Ogwari as other scholars had previously reported heavy metal contamination of numerous water sources in Nigeria (Ibe et al. 2021) and beyond (Vetrimurugan et al. (2017), Enriqueta et al. (2017) Saeed et al. 2014). Mwiathi et al. (2022) investigated the occurrence of geogenic fluoride in shallow aquifers in Kenya. Studies on human health risk exposure to toxic and harmful metals in Pakistan abound (Ali et al., 2021; Iqba et al., 2021; Jehan et al. 2019; Khattak et al. 2021; Noor et al. 2022; Rashid et al. 2021; Rashid et al. 2020; Rashid et al. 2019a; b; Talpur et al. 2020; Ullah et al. 2021). In India, drinking suitability of water resources has been researched extensively (Wagh et al. 2019; Wagh et al. 2020; Kadam et al., 2021; 2022).
Nonetheless, no research of this kind has been attempted on surface and groundwater in Ifite Ogwari, Anambra State, southeast Nigeria and therefore, the need to study exposure risk to heavy metals and its health implications to the residents of Ifite Ogwari community becomes very vital and hence the current study. The aim of the study was to determine the levels of some heavy metals (Ni, Mn, Cr, Cd, Pb, Zn, Fe, and Cu) in surface and groundwater in Ifite Ogwari and analyze the health risks of consuming them by calculating daily oral consumption, hazard quotient (HQ), and total hazard index (THI).
Materials and methods
Study area
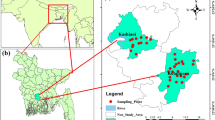
Figure 1 illustrates the study area and sample location points as described in our previous work (Okafor et al 2022a). Briefly, Ifite Ogwari, 45 km from Awka, the capital of Anambra State (Ewuim et al. 2018), is located between latitude 6.6041° North and longitude 6.9507° East, at an elevation of 91 m above sea level (Wikipedia 2018). Ifite Ogwari is located on the banks of the Omambala river and endowed with thick foliage and fertile fields suitable for the growing of food crops such as rice, maize, yam, cassava, okro, and plantain making it a perfect setting for Nnamdi Azikiwe University's Faculty of Agriculture. The overall rainfall averages for the year and month are 5798.78 mm and 1739.62 mm, respectively (Ifeka and Akinbobola 2015). With tropical forests as the dominant vegetation type, the lowest and highest temperatures are 25.4 °C and 30.6 °C, respectively (Iheke and Nwaru 2009; NIMET 2014). The community has a 7-month rainy season (April–October) with a break around July/August and a 5-month dry season (November to March), with harmattan occurring at some points during the dry season. Many rivers and streams run through the area, all of which are part of the Anambra river system which had been identified by Crosskey (1981).
Geologically, Ifite Ogwari is located within the Anambra Basin as depicted in Fig. 2. The Anambra Basin was deposited and filled in two sedimentary phases: transgression and sea regression. The transgression, which occurred during the Campanian—Maastrichtian period, gave rise to deposition of Nkporo Shale, Mamu Formation, Ajalli Sandstone, Nsukka Formation and Imo Shale (Nwajide, 2013; Anakwuba et al., 2021). The study area, Ifite Ogwari is underlain by the Palaeocene Imo Formation (Fig. 2). The Imo Formation is the basal unit of the Niger Delta Basin. The Formation is essentially a mudrock unit consisting of dark gray to bluish gray shale, with occasional admixtures of clay, ironstone, thin sandstone bands, and limestone intercalations (Nwajide, 2013; Anakwuba et al., 2021).
Hydrogeologically, a sand member of the Imo Shale known as the Ebenebe Sandstone usually constitutes of semi-confined to confined aquifers with some boreholes existing under artesian to sub-artesian conditions and greater depths to aquifers (Nwajide, 2013; Anakwuba et al., 2021). The Imo Shale constitutes the aquitard within the study area.
Streams, rivers, and shallow hand-dug wells provide practically all of the water needs for the residents as can be seen in Fig. 3. These people are 100% agriculturists, traditional industrialists, and even civil servants engage in some type of agricultural activity.
Sampling
From Ifite Ogwari, Anambra State, Nigeria, fifteen water samples were collected [9 surface: Isiachala (SA), Iyiutu (SB), Ube (SC), Ahala (SD), Tabasi (SE), Nabaloku (SF), Atammele (SG), Ogbu (SH), and Omambala (RA) and 6 hand-dug wells: Igbazine (WA), Double (WB), Ogba (WC), Onowulugbe (WD), Orator (WE) and Commodore (WF)]. Water sources were chosen for examination because they are available to the community at all times of the year. The sampling sites information is shown in Fig. 2 and Table 1. Samples were collected on February 20, 2020, using previously cleaned glass bottles. At each step of the collection, the bottle was rinsed twice with the water sample to be collected. Streams were designated (SA–SH), river (RA), and hand-dug wells (WA–WF). The water sample sites were geo-referenced using a Garmin GPS map. The samples were packaged and delivered to the Nigerian Institute of Oceanography and Marine Research's Central Laboratory at Victoria Island, Lagos, for heavy metal analysis.
Quality control and heavy metal determination
The analyses required high-quality analytical reagents, which were acquired from BDH Chemical Ltd in the United Kingdom and Sigma-Aldrich Chemie GmbH in Germany. Detergents and deionized water were used to wash the glassware and sample bottles, which were thereafter soaked overnight with a solution of 10% HNO3 in a 1% HCl solution, followed by rinsing with deionized water. Heavy metal analysis by Atomic Absorption Spectrophotometric technique as described by several authors (Assubaie 2015; Ipeaiyeda 2017; Okafor et al. 2022a; b) was adopted and modified. Briefly, 10 cm3 of perchloric acid and 10 cm3 concentrated HNO3 were added to 2 cm3 of the sample in a 250 cm3 beaker. This was boiled in a fume cupboard on a hot plate until white vapors began to emerge. The digestive system was then recharged and heated until white fumes were released. The addition of 20 cm3 of deionized water was then made. The mixture was then boiled for another 20 min until it was particle-free. The digested sample was brought down and cooled to room temperature under the hood. The filtrate was collected in a 50 cm3 volumetric flask after being filtered through No. 11 Whatman filter paper. Before the combined filtrate was made up to mark and placed into a sample container, 20 cm3 of deionized water was used to rinse the filter paper. Standards were made from the salts of the metals to be analyzed, and lamps for the analysis were set up. This was done for Cd, Cr, Cu, Fe, Pb, Mn, Ni, and Zn. The diluents of the sample were aspirated into an Agilent AA500F Atomic Absorption Spectrophotometer.
All of the samples were examined in triplicate, and the metal concentrations were averaged out. The reagent blank, as well as reference material, were analyzed for quality control. During the concentration computation, a blank reading was used to make necessary corrections. The concentrations of the investigated metals indicated in the blank tests were taken into account in the final results for the respective heavy metals. The amount of metal present in each sample was estimated by determining concentration based on absorbance following Beer-Lambert’s law.
Method validation
Limit of detection (LOD), limit of quantification (LOQ), precision, and recovery were evaluated according to the recommendations of the Eurachem Guide (Eurachem 2014). The recommendation, which had previously been utilized by scholars such as dos Santos et al. (2021) and Okafor et al. (2022a) was implemented with adjustments. The analytes’ calibration curves were created in triplicate using deionized water spiked with heavy metal solutions at five concentration levels which were 1.0, 5.0, 10.0, 15.0 and 20.0 µg L−1). LODs and LOQs were determined by analyzing ten blanks. To calculate the LOD, the standard deviation of the replicate's results was multiplied by three and divided by the slope of the calibration curve. The LOQ was computed by multiplying the LOD by 3.33. At concentrations of 5.0 and 15.0 µg L−1, intra-day precision was tested in three replicates at each concentration. On two consecutive days, inter-day precision was examined. Five repetitions were used to assess recovery at a concentration of 10.0 µg L−1.
Heavy metal pollution index
The appropriateness of water is determined by the permissible standards for drinking water set by various organizations and governments. However, a comprehensive understanding of the degree of contamination based on all heavy metals is not possible. As a result, numerous studies have employed the water quality index (WQI) to estimate the overall quality of water based on heavy metals (Horton 1965; Brown et al. 1970).
The WQI values were classified as; WQI < 50 indicates excellent water quality; 50 < WQI ≤ 100 means good water quality; 100 < WQI ≤ 200 indicates poor water quality; 200 < WQI ≤ 300 implies very poor water quality, and WQI > 300 indicates that the water is unfit for consumption (Ramakrishnaiah et al. 2009).
The water quality index was calculated using Eq. (1) (Chatterjee and Raziuddin 2002; Lele et al. 2018).
where Wi is the unit weightage of the ‘i’th heavy metal, n is the number of heavy metals considered and Qi is the sub index of the ‘i’th heavy metal.
The unit weight, Wi, is calculated by
where K is the proportionality constant, Si is the standard permissible limit in water for the ‘i’th heavy metal proportionality constant, K is calculated by,
and
where S1, S2, S3, etc., represent standards for different heavy metals in water such as manganese, cadmium, copper, lead, etc.
The sub index, Qi, is calculated by
where Mi is the monitored value of heavy metal of the ‘i’th heavy metal, Ii is the ideal value of the ‘i’th heavy metal based on international limits for drinking water and Si is the standard value of ‘i’th heavy metal. Table 2 depicts the limits and weight of heavy metals used for WQI calculation.
Multivariate statistical analysis
The utilization of multivariate statistics has the potential for source identification in water quality evaluation in that dataset are analyzed using XL Stat Add-ins for Microsoft ® Excel Package [v 2019] (Omokpariola et al. 2020; Egbueri et al. 2019; Wagh et al. 2018). In this study, Pearson correlation and factor analysis were conducted as 0.30–0.50 (low aggregate), 0.50–0.75 (medium aggregate) and 0.75–1.00 (high aggregate) (Ojaniyi et al. 2021; Barzegar et al. 2019).
Ecological assessment
In this study, several data sets were utilized to evaluate contamination factor (CF), degree of contamination (Deg C), modified degree of contamination (mCDeg), pollution load index (PLI), Nemerow pollution index (NPI) and Potential ecological risk index (PERI).
Contamination factor
Contamination Factor (CF) is the extent of pollution of contaminant of interest, it is expressed as:
The background values of selected heavy metals are Ni = 0.07; Cr = Zn = 0.05; Cd = 0.003; Pb = 0.01; Fe = 0.3; Cu = 2.
Degree of contamination
This is the summation of contamination factor of all chemical contaminants in study site. It is calculated as follows
Modified degree of contamination
This is the average effect of all chemical contaminants of interest, the advantage of mCDeg is that it quantifies the chemical contaminants into a composite aggregate to derive salient information about the study site.
where n is the sum total of chemical contaminant and CF is the contamination factor.
Pollution load index
PLI is the geometric mean of CF value to the nth number of chemical contaminants of interest, it is given as:
where n is the sum total of chemical contaminants and CF is the contamination factor. The PLI gives the level of pollution classified as PLI < 1 (no pollution), 1 < PLI < 2 (modest pollution), 2 < PLI < 3 (high pollution), and 3 < PLI (extremely high pollution).
Nemerow pollution index
It is the complete effect of chemical constituents in the study site which is given as:
where PN is the nemerow pollution index, \(\overline{CF}^{2}\) is arithmetic mean of contamination factor of all chemical contaminants, \({\text{CF}}_{{{\text{max}}}}^{2} \) is the maximum contamination factor among all chemical contaminants. PN is graded as PN < 1 (unpolluted), 1 ≤ PN < 2.5 (slightly polluted), 2.5 ≤ PN < 7 (moderately polluted) and PN > 7 (heavily polluted).
Potential ecological risk index (PERI)
PERI assesses the toxicity factor of a particular chemical contaminant of interest, where the definite contamination status is evaluated in respect to the ecosystem. It is expressed as:
where Er is ecological risk index of different chemical contaminant, TF is toxicity factor of each chemical contaminant of interest, CF is contamination factor in Eq. (1). According to Yi et al. (2017), PERI is graded as PERI < 150 (low risk), 151 < PERI < 300 (moderate risk), 301 < PERI 600 (high risk) and PERI > 320 (very high risk).
Human health risk assessments
Direct ingestion and dermal contact have been suggested as possible human exposure pathways to heavy metal contamination in water. The risk of human exposure from drinking surface and underground water sources was assessed using US EPA risk models for carcinogenic and non-carcinogenic evaluation which is based on Eqs. (12) through (15) (Li and Zhang 2010; Naveedullah et al. 2014; US EPA 1989). The chronic daily intake and hazard index parameter [Eq. (9)] was used to assess the health risk associated with heavy metal absorption through the consumption of surface and groundwater in the research area (Wu et al. 2009; Boateng et al. 2015; Muhammad et al. 2011). CR—ingestion and CR—dermal contact was summed up to get the total cancer risk (Risktotal), while the cumulation of HQ—ingestion and HQ—dermal gave hazard index (HI), as shown in Eqs. (16) and (17)
where CS = heavy metal concentration in water (mg/L), IRw = daily water ingestion rate (L/day) (2.5L/day–adults and 0.78L/day–children) (Brindha et al. 2016), EF = exposure frequency (350-day year−1) (Asare-Donkor et al. 2016), ED = exposure duration (26 years–adults and 6 years–children) (UNDESA 2013), TR = target risk (1 × 106 kg/mg) (US EPA 2020a, b, 2015), CSF = cancer slope factor, BW = body weight (80 kg for adults and 15 kg for children) (ICMR 2009), AT = averaging time (non-carcinogens = ED × 365 days, carcinogen = 70 × 365 days) (Asare-Donkor et al. 2016), RfD = reference dose, SA = skin surface area (19652cm2–adults and 6365cm2–children) (Asare-Donkor et al. 2016). GIABS = fraction of contaminant absorbed in gastrointestinal tracts (unit-less) (1.0 for adults and children) (Naveedullah et al. 2014), RfD and CSF values of some heavy metals are presented in Table 3.
Results and discussion
Quality control and method validation
The equipment used to determine the amounts of heavy metals in the samples (Agilent AA500F) has a high sensitivity—typically > 0.9 absorbance with a precision of < 0.5 percent relative standard deviation (RSD) for a 5 mg/L Cu standard from ten-second integrations. Limits of detection (LOD), limit of quantification (LOQ), repeatability, reproducibility, accuracy, and precision were all used to determine the quality of each water sample. According to the Chinese standard HJ 743–2015, the heavy metal congeners were measured using calibration curves that encompass the dynamic range in which the compounds of interest are expected to be present, and recoveries were extremely good.
Degree of heavy metals contamination in waterbody samples
Figure 4 depicts the semantic differential chart, while Table 4 displays the concentration of heavy metals in water samples from Ifite Ogwari, Anambra State, Nigeria. A detailed examination of the laboratory data reveals that all samples (water bodies) in Ifite Ogwari were free of Cr, Pb and Cu with Ni being the highest in Isiachala, Omambala, and Onowulugbe each having the same concentration of 0.111 mg/L. In 12 sample locations, the concentration of Zn was 0.009 mg/L, while Mn was only found in Iyiutu (0.003 mg/L). Heavy metals are known to be toxic to humans in minute concentrations. The World Health Organization and the United States Environmental Protection Agency provide guideline values for different exposure mediums (oral, dermal, and inhalation) and source pollutants, indicating a propensity to cause adverse health effects (non-cancerous) and cancer-based illnesses for a period of respondents' (adult and children) lifetime (WHO 2017; US EPA 2017). According to the WHO, the tolerable guideline limit for Ni is 0.07 mg/L, indicating that residents of the sample locations (Isiachala, Omambala, and Onowulugbe) may get dermatitis as a result of persistent exposure (WHO 2007). Cd and Zn are known to dissolve with anions (sulfates, chlorides, carbonates), affecting the taste and appearance of water. High concentrations above 0.05 mg/L can cause cytotoxic-induced tumors (cellular mutation) in sensitive organs (kidney and liver) (WHO 2011; 2003). When the human body is poisoned with Cd, Zn is provided as a detoxifying agent to displace and eliminate the Cd through urination or perspiration (Shamelashvili et al. 2020). Furthermore, Fe and Mn are known to affect the color, taste, dissolved oxygen, and turbidity of bodies of water, and microbes use Fe and Mn particulates to make water less palatable and useful for domestic use without the use of a proper treatment or flocculation process (WHO 2011), implying that Fe and Mn have no negative health effects (Xu et al. 2020; Sun et al. 2017).
Multivariate statistical analysis
Correlation analysis of sample locations
The Pearson correlation, shown in Table 5, was conducted for sample locations using the analyzed heavy metal concentration to aggregate the relationship via water body aquifer interactions as Isiachala correlated strongly with Ube, Ahala, Nabaloku, Ogbu, Omambala, Igbazine, Double, Ogba, Orator, and Commodore (0.72–0.99). Iyiutu correlated strongly at 0.86 with Ahala and Igbazine. Nabaloku correlated with Ogbu, Omambala, Igbazine, Double, Ogba, Onowulugbe, Orator, and Commodore, having a range of 0.76–0.99. After assessing a fraction of the correlation across these locations, it is important to note that water is not stationary as it passes through numerous water cycle processes via various environmental strata (atmosphere, hydrosphere, and lithosphere) where there are likely metal releases that are dissipated and concentration levels reduced across multiple water reservoirs.
Factor pattern of sample locations
Principal component analysis was conducted to derive factor patterns across sample location variables using heavy metal concentration levels as revealed in Table 6 and Fig. 5, which produced three factors having a cumulative variance of 33.40%. Factor 1 showed the highest number of locations with aggregates of variance (5.60) in Isiachala, Ube, Ahala, Nabaloku, Ogbu, Omambala, Igbazine, Double, Ogba, Orator, and Commodore with a strong variable range of 0.742–0.986. Factor 2 and 3 produced variance levels of 18.37% and 9.43% with Iyiutu and Atammele in Factor 2 and Tabasi and Onowulugbe in Factor 3.
Correlation analysis of heavy metals
Heavy metals (Ni, Cd, Zn, Fe, and Mn) displayed considerable interaction potentials among studied components, according to Pearson correlation (Table 7) (Okechukwu et al. 2021; Barzegar et al. 2019; 2016; 2017). Positive and negative values were found to have medium (0.50–0.70) and weak (≤ 0.50) correlation matrices, respectively. Natural (rock mineral particulate resuspension) and anthropogenic (industrial emission and releases, automobile, mining, petrochemical) processes are known to interact positively and/or negatively, resulting in a variable increase or decrease in heavy metal concentrations across a variety of environmental mediums (Omokpariola et al. 2020).
Factor pattern of heavy metals
As indicated in Table 8 and Fig. 6, the principal component analysis was extracted using varimax rotation, yielding three factors with a total variance of 79.74%. Factor 1 was responsible for 46.6% in Ni, Fe, and Mn, which could be attributed to anthropogenic rather than natural releases with potentially hazardous concentrations (Utom et al. 2013). In Cd and Fe, factor 2 produced a variation of 21.88%, which can be linked to anthropogenic releases (US EPA 2017). With 11.20% variance, Zn was dominating in Factor 3, indicating that the occurrence might be geogenic and anthropogenic (Bhutiani et al. 2017).
Ecological risk assessment
In Ifite Ogwari, an ecological risk assessment was undertaken to determine the suitability of various water sources for the ecological system. The contamination factor (CF) of various water samples revealed that Ni ranged from 0.41 to 1.59; Cd from 0.00 to 7.67; Zn from 0.00 to 1.66; Fe from 0.00 to 0.28; and Mn from 0.00 to 0.008, with Ni and Cd. CF values ranging from moderate to extremely high levels above the WHO reference standard in some water locations. Cr, Pb, and Cu were not present (no data), and Zn, Fe, and Mn had low CF levels, as reported by Saddique et al. (2018) and Jiao et al. (2015).
For drinking, residential, and industrial applications, the water quality index (WQI) was evaluated (including anthropogenic activities). The computed WQI value ranged from 44.19 to 45.34 and was divided into 3 WQI types: excellent (very fit to drink), good (moderately fit to drink), and poor (unfit to drink) (Brown et al. 1972). The WQI range corresponds to the status level as shown in Table 8 and Fig. 5a, implying that all sample locations are suitable for drinking and household activities such as cooking and bathing, as well as other activities like irrigation. As a result, appropriate water treatment is recommended to raise the WQI level for portable water use in Ifite Ogwari community.
The degree of contamination (Deg-C) and modified degree of contamination (M.Deg-C) are two indicators used to evaluate the effects of ecological pollution because they consider the site's synergistic effect (Vu et al. 2017; Brady et al. 2015). As anthropogenic and natural factors play a vital impact in the level of heavy metal pollution in a given place, modified degree of contamination and degree of contamination are empirical toolkits that measure the level of contamination in a specific sample site (Yan et al. 2016; Duodu et al. 2016). Table 9 and Fig. 7b, c give an adequate classification of the two indices, which shows that M.Deg-C from Isiachala–Tabasi had low contamination, as other locations varied between moderate and high contamination. As regards Deg-C, locations (Isiachala, Iyiutu, Ube, Ogbu, Omambala, Igbazine, Double, Ogba, Onomwulugbe) had a high degree of contamination, as other locations were within low to moderate degrees of contamination.
Other important pollution toolkits used to analyze the effect of heavy metals on the sample sites are the Nemerow pollution index (Yan et al. 2016) and the pollution load index (Ijeh and Onu 2013). Table 8 and Figs. 6a, b show the NPI and PLI indices, with NPI ranging from unpolluted to mildly polluted across all locations, with values ranging from 1.00 to 2.50, and PLI indicating that there was no pollution at all locations. Accordingly, single elemental indices do not provide the same level of insight into synergistic effects as multi-elemental evaluation. As a consequence, the NPI and PLI indices are useful for assessing the combined effects of heavy metals, implying that the water bodies in the Ifite Ogwari community were relatively pollution-free but required additional purification modalities to avoid severe ecological and health impacts to humans, flora, and fauna, respectively.
Another effective method for assessing the likely impact of multi-elemental contamination on the ecology of the Ifite Ogwari community is potential ecological risk indices (PERI). PERI values across all the sample locations are given in Table 9 and Fig. 8c. Using the PERI aggregations (low ecological risk, moderate risk, high ecological risk, and extremely high ecological risk), it is evident that the entire water samples ranged between 5.00 and 238.89, indicating that Ahala, Tabasi, Nabaloku, Atammele, Double, and Onowulugbe were within moderate risk while Isiachala, Iyiutu, Ube, Ogbu, Omambala, and Igbazine were within high risk. Similarly, we can state that immersed anthropogenic activities such as agriculture, mining, construction, and artisanal events have the potential to pose a high ecological risk in the aforementioned locations, even though chemical reactions (dissolution, hydrolysis, redox) can cause an alternating increase and or decrease in heavy metal pollution based on natural phenomena (tidal movement, rock formation, aquifer displacement, chemical mobility, and leaching) in the ecosystem (Tytła 2019; Czaplicka et al. 2017; Xiao et al. 2016, 2015).
Human health risk assessment
Carcinogenic risk assessment
Table 10 and Fig. 9 show the total cancer risk calculated using the cancer slope factor (CSF) from the US Environmental Protection Agency (US EPA) reference guide (mg/kg/day)–1. As can be seen, the overall cancer risk for two respondents (adults and children) was calculated using Ni and Cd. Based on the tolerable cancer risk range of 1.0E–06 to 1.0E–04 (US EPA 2020a, b), the total cancer risk of both adults and children was within a safe range with no carcinogenic health impact (Omokpariola and Omokpariola 2021).
Non-carcinogenic
The evaluation of non-cancer risk was analyzed using different exposure media (ingestion and dermal) and a reference dose (RfD). The sum of exposure mediums was used to determine the cumulative hazard quotient (HQ). The HQ for adults and children was below one (1), as shown in Table 11 and Fig. 10. On the basis of the presented results, the water sources from Ifite Ogwari pose no adverse health risk to the residents.
Except for Okafor et al. (2022a), who studied human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in surface and groundwater from Ifite Ogwari and Ihedioha et al. (2021), who evaluated ecological and human health risks of potential toxic metals in paddy soil, rice plants, and rice grains (Oryza sativa) in the rice field of Omor, a neighboring community to Ifite Ogwari, this study may be the first of its kind in terms of evaluating the exposure risk to heavy metals through surface and groundwater used for drinking and other household activities in Ifite Ogwari and environs. Exposure to heavy metals via drinking water may expose people in such a community to developing cancer at some point in their lives if the water sources are not monitored periodically.
Conclusion
The concentrations of Ni, Cr, Cd, Pb, Zn, Fe, Mn, and Cu in water samples obtained from the major water sources in Ifite Ogwari, namely streams, river, and hand-dug wells, were examined. Heavy metal accumulation in water sources can be hazardous to human health and, in some cases, can lead to life-threatening illnesses, including organ cancer. Ecological indices revealed the ecological impact of multi-element matrices, while health risk assessments revealed that there was no adverse cancer risk or non-cancer risk across respondents (adults and children). The results revealed that anthropogenic discharge has a significant mobility impact on the natural level of metals in Ifite Ogwari water, necessitating effective treatment. The study's findings, which may be used in other places with similar environmental conditions, can serve as a useful benchmark for both local and national governments in developing appropriate approaches to managing surface and groundwater resources.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abrahams PW (2002) Soils: their implication to human health. Sci Total Environ 291(1–3):1–32
Adefemi SO, Awokunmi EE (2009) The impact of municipal solid waste disposal in Ado Ekiti metropolis, Ekiti State, Nigeria. Afr J Environ Sci Technol 3(8):186–189. https://doi.org/10.5897/AJEST09.075
Afiukwa B, Badifu G, Ogunsua AO (2010) Sulphate composition of water from some surface rivers in Nigeria. Plant Foods Human Nutr 41:35–44
Ali L, Rashid A, Khattak SA, Gao X, Jehan S, Javed A (2021) Geochemical modeling, fate distribution, and risk exposure of potentially toxic metals in the surface sediment of the Shyok suture zone, northern Pakistan. Int Jf Sediment Res 36(5):656–667
Anakwuba EK, Okolo CM, Ahaneku VC, Chibuzor SN, Odika NF, Chinwuko AI (2021) Hydrogeophysical evaluation of parts of river Mamu sub-basin, Southeastern Nigeria. Int J Geol Earth Sci 7(1):1–8
Asare-Donkor NK, Boadu TA, Adimado AA (2016) Evaluation of groundwater and surface water quality and human risk assessment for trace metals in human settlements around the Bosomtwe Crater Lake in Ghana. Springer plus 5(1812):1–19
Assubaie FN (2015) Assessment of the levels of some heavy metals in water in Alahsa Oasis farms, Saudi Arabia, with analysis by atomic absorption spectrophotometry. Arab J Chem 8(2):240–245. https://doi.org/10.1016/j.arabjc.2011.08.018
Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M (2021) Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol. https://doi.org/10.3389/fphar.2021.643972
Barzegar R, Moghaddam AA, Tziritis E (2016) Assessing the hydrogeochemistry and water quality of the Aji-Chay River, northwest of Iran. Environ Earth Sci. https://doi.org/10.1007/s12665-016-6302-1
Barzegar R, Moghaddam AA, Soltani S, Fijani E, Tziritis E, Kazemian N (2017) Heavy metalloids in the groundwater of Shabestar area (NW Iran): source identification and health risk assessment. Expo Health. https://doi.org/10.1007/s12403-017-0267-5
Barzegar R, Moghaddam AA, Soltani S, Baomid N, Tziritis E, Adamowski J, Inam A (2019) Natural and anthropogenic origins of selected trace elements in the surface waters of Tabriz area Iran. Environ Earth Sci. https://doi.org/10.1007/s12665-019-8250-z
Bhatia SC (2009) Environmental pollution and control in the chemical process industries. Khanna publishers, India, pp 181–193
Bhutiani R, Kulkarni DB, Khanna DR, Gautam A (2017) Geochemical distribution and environmental risk assessment of heavy metals in groundwater of an industrial area and its surroundings, Haridwar, India. Energ Ecol Environ 2(2):155–167. https://doi.org/10.1007/s40974-016-0019-6
Boateng TK, Opoku F, Acquaah SO, Akoto O (2015) Pollution evaluation, sources, and risk assessment of heavy metals in hand-dug wells from Ejisu-Juaben Municipality. Ghana Environ Syst Res 4(18):1–12
Bolawa OE, Gbenle GO, Ebuehi OAT (2014) Endocrine disruption by the consumption of fish and its reversal using zinc. Int J Aquacul 4:85–88
Brady JP, Ayoko GA, Martens WN, Goonetilleke A (2015) Development of a hybrid pollution index for heavy metals in marine and estuarine sediments. Environ Monit Assess 187(5):1–4
Brindha K, Jagadeshan G, Kalpana L, Elango L (2016) Fluoride in weathered rock aquifers of southern India: managed Aquifer recharge for mitigation. Environ Sci Pollut Res 23:8302–8316
Brown RM, McClelland NI, Deininger RA, Tozer RG (1970) A water quality index—do we dare? Water Sew Works 117(10):339–343
Brown RM, McClelland NI, Deininger RA, O’Connor MF (1972) A water quality index-crashing the psychological barrier. Indic Environ Qual 1(1):173–178
Chatterjee C, Raziuddin M (2002) Determination of water quality index (WQI) of a degraded river in Asansol industrial area (West Bengal). Nat Environ Poll Tech 1(2):181–189
Crosskey RW (1981) A review of Simulium damnosum s. l. and human onchocerciasis in Nigeria with special reference to geographical distribution and development of a Nigerian national control campaign. Tropenmedizin und Parasitologie 32(1):2–16
Czaplicka A, Slusrczyk Z, Szarek-Gwiazda E, Bazan S (2017) Spatial distribution of iron and manganese in bottom sediments of the Goczałkowice Reservoir. Environ Prot 39:47–54
dos Santos RR, Orlando M, Cardeal Z, Menezes HC (2021) Assessment of PAHs and derivatives in beer using a new cold fiber-solid phase microextraction system. Food Control 126:108104. https://doi.org/10.1016/j.foodcont.2021.108104
Duodu GO, Goonetilleke A, Ayoko GA (2016) Comparison of pollution indices for the assessment of heavy metal in Brisbane River sediment. Environ Pollut 219:1077–1091
Egbueri JC, Mgbenu CN, Chukwu CN (2019) Investigating the hydrogeochemical processes and quality of water resources in Ojoto and environs using integrated classical methods. Model Earth Syst Environ 5:1443–1461. https://doi.org/10.1007/s40808-019-00613-y
Enriqueta A, Sergi C, Alexandre R, Ignasi R, Clàudia F (2017) Survey of heavy metal contamination in water sources in the municipality of Torola, El Salvador, through in situ sorbent extraction water. Water 9:877. https://doi.org/10.3390/w9110877
Essien OE, Bassey ED (2012) Spatial variation of borehole water quality with depth in Uyo municipality, Nigeria. Int J Environ Sci Managt Eng Res 1(1):1–9. http://www.ijesmer.com
Eurachem (2014) The fitness for purpose of analytical methods. https://www.eurachem.org/index.php/publications/guides/mv. Accessed Jan 15 2020
Ewuim SC, Ogbuozobe GO, Ezeonyejiaku DC, Mogbo TC (2018) Wet season insect population of an arable land at Ifite-Ogwari campus of Nnamdi Azikiwe University, Awka, Nigeria. Anim Res Int 15(3):3065–3069
Fakayode R (2005) Microbiological and physicochemical analysis of drinking water in Georgetown. Nat Sci 8(8):261–265
Gimeno-García E, Andreu V, Boluda R (1996) Heavy metals incidence in the application of inorganic fertilizers and pesticides to rice farming soils. Environ Pollut 92:19–25. https://doi.org/10.1016/0269-7491(95)00090-9
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37(3):300–306
Ibe FC, Opara AI, Amaobi CE (2021) Ibe BO (2021) Environmental risk assessment of the intake of contaminants in aquifers in the vicinity of a reclaimed waste dumpsite in Owerri municipal Southeastern Nigeria. Appl Water Sci 11:24. https://doi.org/10.1007/s13201-020-01355-4
ICMR (Indian Council of Medical Research) (2009) Nutrient requirements and recommended dietary allowances for Indians. A report of the expert group of the ICMR, Hyderabad, p 334
Ifeka A, Akinbobola, (2015) Trends analysis of precipitation in some selected stations in Anambra State. Atmos Clim Sci 5(1):1–12. https://doi.org/10.4236/acs.2015.51001
Ihedioha JN, Abugu HO, Ujam OT, Ekere NR (2021) Ecological and human health risk evaluation of potential toxic metals in paddy soil, rice plants, and rice grains (Oryza sativa) of Omor Rice Field. Nigeria Environ Monit Assess 193:620. https://doi.org/10.1007/s10661-021-09386-3
Iheke OR, Nwaru JC (2009) Gender farm size and relative productivity of cassava farmers in Ohafia Agricultural Zone of Abia State, Nigeria. Niger J Rural Soc 9(1):69–75
Ijeh BI, Onu NN (2013) Assessment of pollution levels of groundwater in parts of Imo river basin, Southeastern Nigeria. Int J Water Resour Environ Eng 5:194–202
Ipeaiyeda AR (2017) Ayoade AR (2017) Flame atomic absorption spectrometric determination of heavy metals in aqueous solution and surface water preceded by co-precipitation procedure with copper (II) 8-hydroxyquinoline. Appl Water Sci 7:4449–4459. https://doi.org/10.1007/s13201-017-0590-9
Iqbal J, Su C, Rashid A, Yang N, Baloch MYJ, Talpur SA, Ullah Z, Rahman G, Rahman NU, Earjh SMM (2021) Hydrogeochemical assessment of groundwater and suitability analysis for domestic and agricultural utility in Southern Punjab, Pakistan. Water 13(24):3589. https://doi.org/10.3390/w13243589
IRIS from US EPA (US Environmental Protection Agency) (2009) Drinking water standards and health advisories table. https://www3.epa.gov/region9/water/drinking/files/dwshatv
Jehan S, Khan S, Khattak SA, Muhammad S, Rashid A, Muhammad N (2019) Hydrochemical properties of drinking water and their sources apportionment of pollution in Bajaur agency, Pakistan. Measurement 139:249–325. https://doi.org/10.1016/j.measurement.2019.02.090
Jiao X, Teng Y, Zhan Y, Wu J, Lin X (2015) Soil heavy metal pollution and risk assessment in Shenyang industrial district. Northeast China. PLoS ONE 10(5):e0127736. https://doi.org/10.1371/journal.pone.0127736
Kadam A, Wagh V, Patil S, Umrikar B, Sankhua R, Jacobs J (2021) Seasonal variation in groundwater quality and beneficial use for drinking, irrigation, and industrial purposes from Deccan Basaltic Region, Western India. Environ Sci Pollut Res 28:26082–26104. https://doi.org/10.1007/s11356-020-12115-x
Kadam A, Wagh V, Jacobs J, Patil S, Pawar N, Umrikar B, Sankhua R, Kumar S (2022) Integrated approach for the evaluation of groundwater quality through hydro geochemistry and human health risk from Shivganga river basin, Pune, Maharashtra, India. Environ Sci Pollut Res 29:4311–4333. https://doi.org/10.1007/s11356-021-15554-2
Khan AT (2011) Trace elements in drinking water and their possible health effects in Aligarh City. J Water Resource and Prot 3:522–530
Khattak SA, Rashid A, Tariq M, Ali L, Gao X, Ayub M (2021) Potential risk and source distribution of groundwater contamination by mercury in district Swabi, Pakistan: application of multivariate study. Environ Dev Sustain 23:2279–2297. https://doi.org/10.1007/s10668-020-00674-5
Kim KW, Chanpiwat P, Hoang TH, Phan K, Sthiannopkao S (2011) Arsenic geochemistry of groundwater in Southeast Asia. Front Med 5(4):420–433
Lele KC, Verla AW, Amaobi CE, Ajero AI, Enyoh CE, Verla EN (2018) Health risks of consuming untreated borehole water from Uzoubi Umunna Orlu, Imo State Nigeria. J Environ Anal Chem 5(4):1–7
Li SY, Zhang QF (2010) Spatial characterization of dissolved trace elements and heavy metals in the upper Han River (China) using multivariate statistical techniques. J Hazard Mater 176(1–3):579
Masindi V, Muedi KL (2018) Environmental contamination by heavy metals. In: Saleh HEM, Aglan RF (eds) Heavy metals. IntechOpen, London. https://doi.org/10.5772/intechopen.76082
Mitra S, Chakraborty AJ, Tareq AM, Emran TB, Nainu F, Khusro A, Idris AM, Khandaker MU, Osman H, Alhumaydhi FA, Simal-Gandara J (2022) Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J King Saud Univ Sci 34(3):101865. https://doi.org/10.1016/j.jksus.2022.101865
Mudgal SM, Gupta OP, Singh DK, Prasad AS (2010) Comparative physiochemical analysis of river water and underground water in winter season of Rewa town, MP, India. Int Res J Environ Sci 3:59–61
Muhammad S, Shah MT, Khan S (2011) Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Microchem J 98:334–343
Mwiathi NF, Gao X, Li C, Rashid A (2022) The occurrence of geogenic fluoride in shallow aquifers of Kenya Rift Valley and its implications in groundwater management. Ecotoxicol Environ Saf 229:113046. https://doi.org/10.1016/j.ecoenv.2021.113046
Nadal M, Bocio A, Schuhmacher M, Domingo JL (2005) Trends in the levels of metals in soils and vegetation samples collected near a hazardous waste incinerator. Arch Environ Contam Toxicol 49:290–298
Naveedullah MZH, Yu C, Shen H, Duan D, Shen C, Lou L, Chen Y (2014) Concentration and human health risk assessment of selected heavy metals in surface water of the Siling Reservoir watershed in Zhejiang Province, China. Pol J Environ Stud 23:801–811
Nduka JKC, Orisakwe OE, Ezenweke LO, Abiakam CA, Nwanguma CK, Maduabuchi UJ (2006) Metal contamination and infiltration into the soil at refuse dump sites in Awka, Nigeria. Arch Environ Occup Health 61(5):197–204. https://doi.org/10.3200/AEOH.61.5.197-204
Nduka JKC, Orisakwe OE, Ezenweke LO, Chendo MN, Ezenwa TE (2008) Heavy metal contamination of foods by refuse dump sites in Awka, Southeastern Nigeria. Sci World J 8:941–948. https://doi.org/10.1100/tsw.2008.129
NIMET (2014) 2014 Seasonal rainfall prediction (SRP). S and E Consulting, Scottsdale, p 9–15
Nkuma CMA (2000) Standard method for water and effluent analysis, vol 22–23. Foludex press Ltd, Ibadan, pp 44–54
Noor S, Rashid A, Javed A, Khattak JA, Farooqi A (2022) Hydrogeological properties, sources provenance, and health risk exposure of fluoride in the groundwater of Batkhela, Pakistan. Environ Technol Innov 25:102239. https://doi.org/10.1016/j.eti.2021.102239
Nwajide CS (2013) Geology of Nigeria’s sedimentary basins. CSS Bookshop Limited, Lagos, Nigeria
Ojaniyi OF, Okoye PAC, Omokpariola DO (2021) Heavy metals analysis and health risk assessment of three fish species, surface water and sediment samples in Ogbaru axis of river Niger, Anambra State. Nigeria Asian J Appl Chem Res 9(1):64–81. https://doi.org/10.9734/AJACR/2021/v9i130205
Okafor VN, Eboatu AN, Omuku PE (2016) Comparative studies of bitterness, phytochemical and mineral contents of hop extracts and extracts from four selected tropical plants. Asian J Med Health Res 1(6):1–15
Okafor VN, Tabugbo IB, Anyalebechi RI, Okafor UW, Obiefuna JN (2020) A review of Nigerian potential hop substitutes in beer brewing: 1983–2020. Int Res J Pure Appl Chem 21(15):50–73
Okafor VN, Umennadi PU, Odidika CC, Vinna DC (2021) Metals and polycyclic aromatic hydrocarbons (PAHs) in Beer: a review. J Chem Soc Nigeria 46(4):0688–0697. https://doi.org/10.46602/jcsn.v46i4.646
Okafor VN, Omokpariola DO, Okabekwa CV, Umezinwa EC (2022) Heavy metals in alcoholic beverages consumed in Awka, South-East Nigeria: carcinogenic and non-carcinogenic health risk assessments. Chemistry Africa. https://doi.org/10.21203/rs.3.rs-1716686/v1
Okafor VN, Omokpariola DO, Igbokwe EC, Theodore CM, Chukwu NG (2022) Determination and human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in surface and ground waters from Ifite Ogwari, Anambra State, Nigeria. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2022.2038587
Okechukwu VU, Omokpariola DO, Onwukeme VI, Nweke EN, Omokpariola PL (2021) Pollution investigation and risk assessment of polycyclic aromatic hydrocarbons in soil and water from selected dumpsite locations in Rivers and Bayelsa State Nigeria. Environ Anal Health Toxicol 36(4):e2021023. https://doi.org/10.5620/eaht.2021023
Omokpariola DO (2021) Experimental Modelling Studies on the removal of crystal violet, methylene blue and malachite green dyes using Theobroma cacao (Cocoa Pod Powder). J Chem Lett 2:9–24. https://doi.org/10.22034/jchemlett.2021.272842.1020
Omokpariola DO, Omokpariola PL (2021) Health and exposure risk assessment of heavy metals in rainwater samples from selected locations in Rivers State, Nigeria. Phy Sci Rev 0090:1–14. https://doi.org/10.1515/psr-2020-0090
Omokpariola DO, Nduka JK, Omokpariola PL, Omokpariola ECO (2020) Ionic composition of rainwater from different sampling surfaces across selected locations in Rivers State, Nigeria. World Sci News 150:132–147
Orakwue FC, Okafor VN, Obumselu FO, Okoli JO, Nnamdi KC (2021) Phytochemical, antimicrobial and human health risk assessement of heavy metals of stem and root extracts of Newbouldia laevis (boundary tree). J Chem Soc Nigeria 46(6):0940–0950. https://doi.org/10.46602/jcsn.vi5.673
Ramakrishnaiah CR, Sadashivaiah C, Ranganna G (2009) Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka State, India. E-J Chem 6(2):523–530
Rashid A, Khattak SA, Ali L, Zaib M, Jehan S, Ayub M, Ullah S (2019a) Geochemical profile and source identification of surface and groundwater pollution of District Chitral, Northern Pakistan. Microchem J 145:1058–1065. https://doi.org/10.1016/j.microc.2018.12.025
Rashid A, Farooqi A, Gao X, Zahir S, Noor S, Khattak JA (2020) Geochemical modeling, source apportionment, health risk exposure and control of higher fluoride in groundwater of sub-district Dargai Pakistan. Chemosphere 243:125409. https://doi.org/10.1016/j.chemosphere.2019.125409
Rashid A, Ayub M, Javed A, Khan S, Gao X, Li C, Ullah Z, Sardar T, Muhammad J, Nazneen S (2021) Potentially harmful metals, and health risk evaluation in groundwater of Mardan, Pakistan: application of geostatistical approach and geographic information system. Geosci Front 12(3):101128. https://doi.org/10.1016/j.gsf.2020.12.009
Sada PO, Odemerho FO (1988) Environmental issues and management in Nigerian development. Evans brothers, Ibadan, pp 67–75
Saddique U, Muhammad S, Tariq M, Zhang H, Arif M, Jadoon IAK, Khattak NU (2018) Potentially toxic elements in soil of the Khyber Pakhtunkhwa province and Tribal areas, Pakistan: evaluation for human and ecological risk assessment. Environ Geochem Health. https://doi.org/10.1007/s10653-018-0091-2
Saeed S, Marzieh VD, Akbar H, Toba K (2014) Heavy metals in water and sediment: a case study of Tembi river. J Environ Public Health. https://doi.org/10.1155/2014/858720
Sarker A, Kim JE, Islam ARMT, Bilal M, Rakib MRJ, Nandi R, Rahman MM, Islam T (2022) Heavy metals contamination and associated health risks in food webs—a review focuses on food safety and environmental sustainability in Bangladesh. Environ Sci Pollut Res 29:3230–3245. https://doi.org/10.1007/s11356-021-17153-7
Shamelashvili K, Ostrovska S, Shatorna V (2020) The toxic effect of cadmium on living organism and its detoxification by zinc ions. Mod Sci 3:150–157
Stark JR, Hanson PE, Goldstein RM, Fallon JD, Fong AL, Lee KE, Kroening SE, Andrews WJ (2001) Quality in the upper Mississippi River basin, Minnesota, Wisconsin, South Dakota, Iowa, and North Dakota 1995–98. United States Geological Survey, Reston
Stiglian WM, Doelman P, Salomons W, Scjulin R, Schmidt GRB (1991) Chemical time bombs-predicting the unpredictable. Environment 33:4–30
Sun H, Shi B, Yang F, Wang D (2017) Effects of sulfate on heavy metal release from iron corrosion scales in drinking water distribution system. Water Res 114:69–77
Talpur SA, Noonari TM, Rashid A, Ahmed A, Baloch MYJ, Talpur HA, Soomro MH (2020) Hydrogeochemical signatures and suitability assessment of groundwater with elevated fluoride in unconfined aquifers Badin district, Sindh Pakistan. SN Appl Sci 2:1038. https://doi.org/10.1007/s42452-020-2821-1
Tytła M (2019) Assessment of heavy metal pollution and potential ecological risk in sewage sludge from municipal wastewater treatment plant located in the most industrialized region in Poland–case study. J Environ Res Public Health 16:2430
Ullah Z, Talib MA, Rashid A, Ghani J, Shahab A, Irfan M, Rauf A, Bawazeer S, Almarhoon ZM, Mabkhot YN (2021) Hydrogeochemical investigation of elevated arsenic based on entropy modeling, in the aquifers of district Sanghar, Sindh Pakistan. Water 13(23):3477. https://doi.org/10.3390/w13233477
UNDESA (United Nations Department of Economic and Social Affairs) (2013) World population prospects. Population Division Database. Detailed indicators 2012 Revision
United States Environmental Protection Agency (US EPA) (2000) Risk-based concentration table. United States Environmental Protection Agency, Philadelphia, Washington
US Epa (United States Environmental Protection Agency) (2000) Handbook for non-cancer health effects evaluation. US Environmental Protection Agency, Washington DC
US EPA (2020b) Human health risk assessment: Risk-based concentration table. Regional Screening Levels (RSLs) Summary Table (TR=1E-06, HQ=1). https://www.epa.gov/risk/regional-screening-levels-rsls-generictables. Assessed on Nov 2020
US EPA (1989) Risk assessment guidance for superfund, vol 1: human health evaluation manual (part A). Office of Emergency and Remedial Response, Washington
US EPA (2007) Framework for metals risk assessment. US Environmental Protection Agency, Washington
US EPA (2011) Exposure Factors Handbook Final. National Center for Environmental Assessment Office of Research and Development, Washington
US EPA (2017) National Recommended Water Quality Criteria—Aquatic Life Criteria Table and Human Health Criteria Table. United States Environmental Protection Agency, Washington DC
US EPA (2020) Regional Screening levels (RSLs) Table. United States Environmental Protection Agency, Washington DC
US Epa (2015) Recommended use of BW3/4 as the default method in derivation of the oral reference dose. US Environmental Protection Agency, Washington
Utom AU, Odoh BI, Egboka BCE (2013) Assessment of hydrogeochemical characteristics of groundwater quality in the vicinity of Okpara coal and Obwetti fireclay mines, near Enugu town, Nigeria. Appl Water Sci 3:271–283
Vetrimurugan E, Brindha K, Elango L, Osman MN (2017) Human exposure risk to heavy metals through groundwater used for drinking in an intensively irrigated river delta. Appl Water Sci 7:3267–3280. https://doi.org/10.1007/s13201-016-0472-6
Viridor Waste Ltd (2009) Viridor New England energy from waste project: technical data for HHRA generic assessment criteria (402–0036–00350). http://www.devon.gov.uk/plandoc259_4975.pdf. Accessed 1 July 2016
Vu CT, Lin C, Shern CC, Yeh C, Le VG, Tran HT (2017) Contamination, ecological risk and source apportionment of heavy metals in sediments and water of a contaminated river in Taiwan. Ecol Ind 82:32–42. https://doi.org/10.1016/j.ecolind.2017.06.008
Wagh VM, Panaskar DB, Mukate SVG, SK, Muley AA, Varade AM, (2018) Health risk assessment of heavy metal contamination in groundwater of Kadava river basin, Nashik, India. Model Earth Syst Environ 4:969–980. https://doi.org/10.1007/s40808-018-0496-z
Wagh VM, Mukate SV, Panaskar DB, Muley AA, Sahu YL (2019) Study of groundwater hydrochemistry and drinking suitability through water quality index (WQI) modelling in Kadava river basin. India SN Appl Sci 1:1251. https://doi.org/10.1007/s42452-019-1268-8
Wagh V, Mukate S, Muley A, Kadam A, Panaskar D, Varade A (2020) Study of groundwater contamination and drinking suitability in basaltic terrain of Maharashtra, India through PIG and multivariate statistical techniques. J Water Supply Res Technol AQUA 69(4):398–414. https://doi.org/10.2166/aqua.2020.108
WHO (2003) Zinc in drinking-water? Background document for preparation of WHO Guidelines for drinking-water quality. WHO, Geneva
WHO (2007) Nickel in drinking-water? Background document for development of WHO guidelines for drinking-water quality. WHO, Geneva
WHO (2011) Cadmium in drinking-water? Background document for preparation of WHO guidelines for drinking-water quality. WHO, Geneva
WHO (2017) Guidelines for drinking-water quality: fourth edition incorporating the first addendum? World Health Organization, Geneva
Wikipedia (2018) Orthetrum icteromeles, http://en.m.wikipedia.org. Accessed Aug 8 2021.
Wu B, Zhao DY, Jia HY, Zhang Y, Zhang XX, Cheng SP (2009) Preliminary risk assessment of trace metal pollution in surface water from Yangtze river in Nanjing section. China Bull Environ Contam Toxicol 82(4):405–409
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risk and best available strategies for remediation. Int Sch Res Not. https://doi.org/10.5402/2011/402647
Xiao Z, Yuan X, Li H, Jiang L, Leng L, Chen X, Zeng G, Li F, Cao L (2015) Chemical speciation, mobility and phyto-accessibility of heavy metals in fly ash and slag from combustion of pelletized municipal sewage sludge. Sci Total Environ 536:774–783. https://doi.org/10.1016/j.scitotenv.2015.07.126
Xiao Z, Yuan X, Leng L, Jiang L, Chen X, Zhibin W, Xin P, Jiachao Z, Zeng G (2016) Risk assessment of heavy metals from combustion of pelletized municipal sewage sludge. Environ Sci Pollut Res 23:3934–3942. https://doi.org/10.1007/s11356-015-5213-0
Xu X, Shuming L, Smith K, Ciu Y, Wang Z (2020) An overview on corrosion of iron and steel components in reclaiming water supply systems and the mechanism involved. J Clean Prod. 276:124079. https://doi.org/10.1016/j.jclepro.2020.124079
Yan N, Liu W, Xie H, Gao L, Han Y, Wang M, Li H (2016) Distribution and assessment of heavy metals in the surface sediment of Yellow River, China. J Environ Sci 39:45–51. https://doi.org/10.1016/j.jes.2015.10.017
Yi, XU, Liang X, Yingming XU, Xu QIN, Huang Q, Lin W, Yuebing S (2017) Remediation of heavy metal-polluted agricultural soils using clay minerals: a review. Pedosphere 27(2):193–204. https://doi.org/10.1016/S1002-0160(17)60310-2
Acknowledgements
The authors would like to thank the technical staff of the Central Laboratory, Nigerian Institute for Oceanography and Marine Research, Victoria Island, Lagos, most especially Macaulay Iduma, for technical assistance.
Funding
This research was funded by the authors.
Author information
Authors and Affiliations
Contributions
Project conceptualization, investigation, methodology, and the first draft of the manuscript (Vincent N. Okafor). The final text of the manuscript, as well as literature searches and data curation (Vincent N. Okafor, Daniel O. Omokpariola, Onyeka F. Obumselu and Chiadikaobi G. Eze). All of the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okafor, V.N., Omokpariola, D.O., Obumselu, O.F. et al. Exposure risk to heavy metals through surface and groundwater used for drinking and household activities in Ifite Ogwari, Southeastern Nigeria. Appl Water Sci 13, 105 (2023). https://doi.org/10.1007/s13201-023-01908-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-01908-3