Abstract
Karoon River is the most important river in Iran that supplies the drinking and industrial water to many cities and villages in Khuzestan. As a consequence of hospital and municipal wastewater discharge into this river, antibiotic resistance will be increased in microbial inhabitants of this ecosystem. Furthermore, microbial load is also undergone continual variation. The aim of this study was to evaluate seasonal microbial quality and antibiotic resistance in bacterial isolates. For this purpose, five stations were selected and samples were harvested during four seasons. Bacterial count was performed through culturing on Mueller–Hinton agar, and bacterial isolates were identified. Antibiotic resistance profiles of isolates as well as resistance to Hg were investigated. As a result, it was found that the least quality was in summer season while the best quality was in winter that is related to the reduction of water volume and recreational activities in summer and increasing rain and dilution of contaminants in winter season. All isolates were sensitive to Hg while antibiotic resistance and multidrug resistance were found in bacterial isolates. Based on the obtained results, it can be concluded that the microbial quality of Karoon River has variations depending on the season and it is necessary that be monitored in order to control and prevent epidemic bacterial infections and antibiotic resistance distribution.
Similar content being viewed by others
Introduction
Historically, humans were resided around the rivers and following this, cities, agriculture and industries were developed near the rivers. Rivers are one of the most vital sources for water supplying for humans (Sánchez et al. 2007; Simeonov et al. 2003). Parallel to population growth, the water demand has been increased. Furthermore, due to increase in agricultural and industrial activities and water pollution, the available water resources have been limited. Therefore, management and quality control of water resources is necessary (Fataei 2012). Karoon River is the longest river in Iran that supplies 70% of drinking water of Khuzestan Province. This river is contaminated with different wastewaters, and any interruption in its disinfection will lead to contamination and infection in peoples. Presently, high volume of agricultural drainages and industrial, aquaculture and municipal wastewaters are discharged to this river, which reduces the water quality, especially in low-rain seasons (Madadi Nia et al. 2014). Heavy metals and antibiotics are important contaminants that are introduced with these wastewaters into Karoon River (Audi 1994; Mara and Horan 2003). Heavy metals can be accumulated in food chain, and hence this leads to human toxicity. In addition, these contaminants will lead to the selection of heavy metal-resistant microorganisms that are health hazards for humans and animals (Johnson et al. 2007). Antibiotics are routinely used for treatment of bacterial infections in medicine as well as veterinary medicine. Municipal, hospital, animal farms and aquaculture wastewaters are the main sources of antibiotics that are directly introduced into Karoon River and consequently will increase antibiotic-resistant microorganisms in environment. Such microorganisms can cause infections that are resistant to treatment or during horizontal gene transfer, will donate resistance genes to pathogenic bacteria, and hence this can cause even life-threatening infections (Kümmerer 2004; Rahube and Yost 2010). With regard to this fact, frequent monitoring of the Karoon River water quality is vital for prevention of the undesirable outcomes of water contamination. The aim of the present study was to investigate water quality of Karoon River in different seasons and determine the antibiotic resistance profile of bacterial isolates. These data are useful guidelines for control and prevention of infectious disease epidemics and restriction of the distribution of antibiotic resistance among bacterial species.
Materials and methods
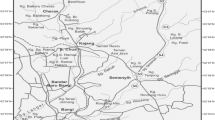
Water samples were harvested from five selected stations along the Karoon River (Table 1). Samples were transported to laboratory on ice in sterile glass bottles and stored at 4 °C till laboratory experiments. Water temperature was recorded on site. Sampling was repeated in the middle of each season.
Physical and chemical parameters of water samples including pH, electrical conductivity (EC), dissolved oxygen (DO), chemical oxygen demand (COD) and biological oxygen demand (BOD5) were determined just following to samples delivery to laboratory (Ur Rehman et al. 2017).
Serial tenfold dilutions were prepared from each sample, and 100 µl from 10−4 dilution was cultured on Mueller–Hinton agar, Cetrimide agar, MacConkey agar and EMB agar and incubated at 30 °C for 24 h. The grown colonies on Mueller–Hinton agar were counted and reported as total viable count. Furthermore, the colonies appeared on other media were counted and identified through standard biochemical, morphological and enzymatic tests. The antibiotic susceptibility of purified isolates was investigated according to Kirby-Bauer disk diffusion method using CLSI guidelines 2010 (Wayne 2010). For this purpose, each bacterial isolate was inoculated in Mueller–Hinton broth and incubated at 37 °C till 0.5 McFarland turbidity growth. Then, using a sterile swab, a lawn culture was prepared on Mueller–Hinton agar and standard antibiotic disks including gentamicin, tetracycline, penicillin, cefoxitin, erythromycin, trimethoprim sulfamethoxazole, ciprofloxacin and cephalexin were placed on the prepared culture. The plates were incubated at 37 °C for 24 h, and the formed halo zone around each disk was measured (mm) and recorded. The obtained data were interpreted based on the guidelines presented by CLSI 2010 (Wayne 2010). In order to determine the susceptibility of isolates to Hg, three different concentrations of HgSO4, i.e., 0.05, 0.03 and 0.01 g/ml, were prepared in distilled water and sterile blank disks were saturated with these solutions. The susceptibility of isolates to these different concentrations of Hg was investigated according to what described for antibiotic susceptibility test (Manegabe 2015; Mutuku et al. 2014).
Results
As mentioned in the previous section, five different stations were sampled in four seasons. Table 2 presents the season temperature during sampling. Evaluation of physical and chemical parameters of the water samples including pH, EC, DO, COD, BOD5 and the results of their counting bacteria in different cultures are presented in Tables 3, 4 and 5.
The acceptable pH for drinking water is between 6.5 and 8.5. As we can see, in all seasons the pH was in acceptable range (WHO 2011). The pH of the samples collected in summer was higher than other seasons. This can be due to the reduction in rain and the volume of water in river. However, the addition of wastewaters and agricultural drainages can reduce the pH of Karoon River. The DO content of all stations was higher in winter and autumn than in other seasons except station 2 that its DO content in summer and autumn seasons was more than that in other seasons. The highest content of DO was recorded in station 1 in all seasons. As this station is located at the upstream part of Karoon River, it seems that less contamination had been happened in this station and hence it had higher DO. The least DO was related to spring season in all stations, and among the stations, station 5 showed the least DO. Overall, the DO content was reduced in spring and summer seasons that can be as a consequence of reduction in rain and increase in the salts content of water. Furthermore, parallel to increasing the temperature, the activity of microorganisms will be increased that leads to the reduction of DO. On the other hand, with decreasing the temperature and increasing the rain in autumn and winter seasons, the water flood and its DO will be increased (Wayne 2010; Hajizadeh Zaker and Eghtesadi Araghi 2009). In case of BOD5, the least BOD5 in all stations was recorded at winter and spring seasons except in station 1 that the least BOD5 was found in spring and autumn seasons. In stations 3, 4 and 5, the BOD5 content in water samples in autumn season was higher than other seasons. The highest BOD5 was related to station 5 in summer, and the least was recorded from station 1 in spring season. As station 5 is located in the downstream of Ahvaz city, the reason for increase of BOD5 is related to municipal wastewater discharges and agricultural drainages into Karoon at this station. In all stations, the highest COD was recorded in autumn. The highest COD was related to station 5 in autumn season, and station 1 had the least COD in winter season. This pattern of this parameter in these stations is similar to BOD5. In bacterial count, it was found that in spring and summer seasons, high microbial load was present in Karoon River. The majority of identified species from these samples were from Enterobacteriaceae family (including Escherichia coli, Salmonella, Shigella, Klebsiella, Enterobacter) and Pseudomonas aeruginosa. In all stations, the highest contamination with P. aeruginosa, E. coli and Klebsiella sp. was recorded in summer season and the least contamination with these bacterial species was in winter, autumn and autumn seasons, respectively. Contamination with Shigella sp., Salmonella sp. and Enterobacter sp. was high in spring season and low in autumn season. As in summer season, the environmental temperature is more favorable for growth of these bacteria and the highest bacterial number was recorded in summer season. The order of bacterial count in all stations was as follows:
-
Enterobacter sp. < Salmonella sp. < E. coli < Shigella sp. < P. aeruginosa < Klebsiella sp.
Fortunately, all bacterial isolates were sensitive to Hg and no resistant species was found. The results of antibiotic susceptibility test are presented in Table 6.
The total diversity number of isolated bacteria in autumn, winter, spring and summer was 14, 29, 38, and 40, respectively. Multidrug resistance has been found in many of bacterial isolates (Table 7). This is due to abuse of antibiotic therapy in human and animals. The highest number of bacterial isolates was resistant to cephalexin, and the least number of resistances was found against ciprofloxacin.
Discussion
Water quality is one of the important factors for drinking water; hence, water can act as a vector for transmission of pathogens to humans and animals. So, monitoring of water quality and prevention from its contamination is one of the key steps in supplying drinking water. Karoon River is a main source of water supply in Khuzestan so maintaining its quality is of great importance. The results of this research showed that there is high microbial count in the sampled waters from Karoon; however, dependent on the season and station of sampling, this microbial load was varied. With regard to these results, it can be concluded that the discharge of different wastewaters and agricultural drainage to this river had adverse effects on the quality of water in such a manner that the worst quality was recorded in station 5 that is located in downstream of Ahvaz city. The high microbial count, BOD5 and COD and low DO in this station explained that the addition of industrial, municipal and aquaculture wastewaters during its crossover from Ahvaz has led to higher contamination and lower quality. In other studies, on this river, similar results have been reported. Shariat et al. (2006) revealed that the water quality in upstream stations is more favorable than downstream stations and in most stations the best and the worst quality was recorded in winter and spring seasons, respectively (Shariat et al. 2006). In the present study, the best quality was in winter and autumn and the worst quality was in summer and spring seasons. Roshanfekr et al. (2006) reported that water quality of Karoon River was best in winter and worst in summer season (Roshanfekr et al. 2006). In the study of Madadi Nia et al. (2014), it was reported that the water quality was reduced from upstream stations toward downstream stations. Furthermore, the autumn season had the best quality while the spring showed the worst quality (Madadi Nia et al. 2014). Chaurasia and Tiwari (2011) in a study on the water quality on Rapti River in India reported that due to discharge of sugar factories wastewater into this river, the water quality was reduced (Chaurasia and Tiwari 2011). Edokpayi et al. (2015) evaluated the water quality of Mvudi River in South Africa and compared the bacterial count in two dry and wet seasons. Their results showed that there was no significant difference in E. coli number between two seasons (Edokpayi et al. 2015). Similarly, in the present study, there was no significant difference between four seasons in case of E. coli count. However, the highest contamination was recorded in summer (dry) season. Soko and Gyedu-Ababio (2015) found that human activities like agriculture, industry and also cities development had destructive effects on water quality (Soko and Gyedu-Ababio 2015). These results are in agreement with the obtained results in the present study. Sair and Khan (2018) determined microbial diversity, distribution of antibiotic and metal resistance of isolated bacteria from five rivers. The resistance of the studied isolates which was tested against ten antibiotics and five metals indicated high resistance of the bacterial isolates against ampicillin, streptomycin and chloramphenicol. Isolates showed tolerance to different concentrations of heavy metals. High antibiotic resistance and metal resistance in samples from the Ravi and Soan Rivers suggest co-resistance among the bacterial populations (Sair and Khan 2018). Proia et al. (2018) described the prevalence of antibiotic resistance of E. coli and freshwater bacteria over a 1-year period along sewage-polluted sites of Zenne River (Belgium). Resistant E. coli and heterotrophic bacteria to amoxicillin, sulfamethoxazole, nalidixic acid and tetracycline were determined according to culture-dependent methods. The obtained results showed an effect of treated wastewaters release on the spread of antibiotic resistance along the river (Proia et al. 2018).
Korrapati et al. (2010) isolated bacterial strains from samples collected from the oxidation pond stage of wastewater treatment of NIT Warangal and identified their resistance. In this study, the maximum and minimum metal tolerance was shown for chromium and nickel, respectively. The most antibiotic resistance was shown for amoxicillin while the least resistance was observed against kanamycin. This study stated that the metal-resistant strains can be used for treating wastewater effluents from industrial or domestic sources and bioremediation process (Korrapati et al. 2010). Rai et al. (2010) analyzed water samples from sewage treatment plants which are regularly discharged into the Ganga River. Biological oxygen demand and dissolved oxygen values were above the standard limit at all sites. Heavy metals (Zn, Cu, Cd, Pb, Cr) in disposed effluents were above permissible limits at all sites. This study recommended an integrated approach of phytoremediation with aquatic macrophytes and ozonization of wastewater to curb the heavy metals and microbial pollution (Rai et al. 2010). Graham et al. (2010) quantified 13 antibiotic resistance genes (ARG; indicators of AR potential), six heavy metals, three antibiotics and 17 other organic pollutants at eight locations along the Almendares River in western Havana at sites bracketing known waste discharge points, including a large solid waste landfill and various pharmaceutical factories. Significant correlations (p < 0.05) were found between sediment antibiotic resistance gene (ARG) levels, especially for tetracyclines and β-lactams [e.g., tet(M), tet(O), tet(Q), tet(W), blaOXA], and sediment Cu and water column ampicillin levels in the river. Further, sediment ARG levels increased by up to three orders of magnitude downstream of the pharmaceutical factories and were highest at high human population density. These results suggest that pollution has increased the background AR levels in situations where other causes of AR are less prevalent (Graham et al. 2010). Seiler and Berendonk (2012) reviewed the co-selection of heavy metals for antibiotic resistance in soil and water and their mechanisms, the available data on heavy metal pollution, heavy metal toxicity and heavy metal tolerance. In this study, analyses of the data indicated that agricultural and aquacultural practices were major sources of soil and water contamination with toxic metals such as mercury (Hg), cadmium (Cd), copper (Cu) and zinc (Zn) and if those metals reach the environment and accumulate to critical concentrations, they can trigger co-selection of antibiotic resistance. This study suggested focusing on Hg, Cd, Cu and Zn as selecting heavy metals in studies which investigating the co-selection in environments impacted by agriculture and aquaculture (Seiler and Berendonk 2012).
Conclusion
According to the obtained data, it can be explained that Karoon River is undergoing frequent quality change and there is a risk of antibiotic resistance distribution among bacterial populations of this ecosystem. So effective precautions must be made to prevent untreated wastewater discharge to this river and applying biofiltration for antibiotic biodegradation prior to its release in Karoon River.
References
Audi G (1994) The quality of drinking water. Mohaghegh, Mashahad, pp 19–26 (in Persian)
Chaurasia NK, Tiwari RK (2011) Effect of industrial effluents and wastes on physico-chemical parameters of river Rapti. Adv Appl Sci Res 2(5):207–211
Edokpayi JN, Odiyo JO, Msagati TA, Potgieter N (2015) Temporal variations in physico-chemical and microbiological characteristics of Mvudi River, South Africa. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph120404128
Fataei A (2012) A survey on quality parameters of Pars Abad water supply channels. Environ Sci Eng 50:72–81 (In Persian with English abstract)
Graham DW, Olivares-Rieumont S, Knapp CW, Lima L, Werner D, Bowen E (2010) Antibiotic resistance gene abundances associated with waste discharges to the Almendares River near Havana, Cuba. Environ Sci Technol. https://doi.org/10.1021/es102473z
Hajizadeh Zaker N, Eghtesadi Araghi P (2009) Characteristics and seasonal variations of pH in the Southern Shelf of the Caspian Sea. J Environ Stud 35(50):19–26 (In Persian)
Johnson RL, Redding K, Holmquist DD (2007) Water quality with Vernier. Vernier Software and Technology, Beaverton
Korrapati N, Rao PS, Vinod AV (2010) Isolation and identification of bacterial strains and study of their resistance to heavy metals and antibiotics. J Microb Biochem Technol 2(3) (1000027 ref. 14)
Kümmerer K (2004) Resistance in the environment. J Antimicrob Chemother. https://doi.org/10.1093/jac/dkh325
Madadi Nia M, Monavari M, Karbasi A, Nabavi MB, Rajabzadeh E (2014) Study on water quality of Karoun River (Ahvaz region) using water quality index. J Environ Sci Technol 16(1):49–60 (In Persian with English abstract)
Manegabe BJ (2015) Assessment of pathogenic bacteria and heavy metal pollution in sediment and water of Kahwa River, Bukavu, Democratic Republic of the Congo. Doctoral dissertation
Mara D, Horan NJ (eds) (2003) Handbook of water and wastewater microbiology. Elsevier, New York City
Mutuku C, Okome P, Boga H (2014) Association of metal tolerance with multidrug resistance among environmental bacteria from wetland of Lake Victoria basin. Agric Biol J N Am 5:24–32
Proia L, Anzil A, Subirats J, Borrego C, Farrè M, Llorca M et al (2018) Antibiotic resistance along an urban river impacted by treated wastewaters. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2018.02.083
Rahube TO, Yost CK (2010) Antibiotic resistance plasmids in wastewater treatment plants and their possible dissemination into the environment. Afr J Biotech 9:9183–9190
Rai PK, Mishra A, Tripathi BD (2010) Heavy metal and microbial pollution of the River Ganga: a case study of water quality at Varanasi. Aquat Ecosyst Health Manage. https://doi.org/10.1080/14634988.2010.528739
Roshanfekr A, Tavakolizadeh A, Kashefipour A (2006) Quality assessment of Karoon River water using water quality indices for application in irrigation networks. In: National conference of water and drainage network management 2–4 May, Ahvaz, Iran (in Persian)
Sair AT, Khan ZA (2018) Prevalence of antibiotic and heavy metal resistance in Gram negative bacteria isolated from rivers in northern Pakistan. Water Environ J. https://doi.org/10.1111/wej.12290
Sánchez E, Colmenarejo MF, Vicente J, Rubio A, García MG, Travieso L, Borja R (2007) Use of the water quality index and dissolved oxygen deficit as simple indicators of watersheds pollution. Ecol Ind. https://doi.org/10.1016/j.ecolind.2006.02.005
Seiler C, Berendonk TU (2012) Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front Microbiol. https://doi.org/10.3389/fmicb.2012.00399
Shariat M, Jafarzadeh N, Sakiyan M, Fadaei AN (2006) Determination of Dez River water quality using Shahrekord Water Quality Indicator Curve. In: Regional conference on optimal utilization of water resources in Karoon and Zayandeh rood basins, vol 1, no 1 (in Persian)
Simeonov V, Stratis JA, Samara C, Zachariadis G, Voutsa D, Anthemidis A et al (2003) Assessment of the surface water quality in Northern Greece. Water Res. https://doi.org/10.1016/S0043-1354(03)00398-1
Soko MI, Gyedu-Ababio T (2015) The spatial and temporal variations of ichythyofauna and water quality in the Crocodile River (East), Mpumalanga, South Africa. J Water Resource Prot. https://doi.org/10.4236/jwarp.2015.73013
Ur Rehman U, Khan A, Shah MT, Muhammad J, Nasir MJ, Khan S (2017) Quantification of essential elements, their daily intake and health risk via drinking water collected from Southern Khyber Pakhtunkhwa, Pakistan. J Himal Earth Sci 50(2):14–26
Wayne PA (2010) Clinical and laboratory standards institute: performance standards for antimicrobial susceptibility testing: 20th informational supplement. CLSI document M100-S20
World Health Organization (2011) Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8–11 December 2008
Acknowledgements
The authors wish to thank vice chancellor of research of Shahid Chamran University of Ahvaz for providing research grant (Grant No. 874095) and MSc. thesis grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Besharati, S., Motamedi, H. & Zallaghi, R. A survey on microbial quality and antibiotic resistance in Karoon River, Khuzestan, Iran. Appl Water Sci 8, 146 (2018). https://doi.org/10.1007/s13201-018-0786-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0786-7