Abstract
The sediments in stormwater runoff are recognised as the major sink of the heavy metals and affect the soil quality in the catchment. The runoff sediments are also important in the management of contaminant transport to receiving water bodies. In the present work, stormwater during several major rain events was collected from nine principal locations of Guwahati, India. The solid phase was separated from the liquid phase and was investigated for the total contents of Cd, Co, Cr, Cu, Mn and Zn as well as their distribution among the prominent chemical phases. Sequential extraction procedure was used for the chemical fractionation of the metals that contains five steps. The total metal concentration showed the trend, Cd < Co < Cu < Cr < Zn < Mn. The relative distribution of the metals showed that Cd was available mostly in the exchangeable and the carbonate bound fractions, which were the most mobile and high-risk fractions. Co with medium mobility was also found to be in the high-risk category. On the other hand, the mobilities of Cu and Zn were relatively low and these were, therefore, the least bioavailable metals in the runoff sediments falling in medium-risk category.
Similar content being viewed by others
Introduction
The stormwater sediments form an important sink for the contaminants emitted from vehicles, accumulation of garbage, effluents from households and various civic amenities, industrial production and urban constructions (Mahanta and Bhattacharyya 2011; Huang et al. 2013). The metal contamination of soil may also be a source of landscape change, habitat destruction, soil and water contamination and land resource degradation. Heavy metals in soils are toxic to plants, animals and humans via the water and the food chain (Huang et al. 2013). It is important to measure the pollutant loads of the sediments and the mobility characteristics under different chemical environments (Violante et al. 2010). The contamination of urban sediments by heavy metals is a major concern now-a-days due to the possibility of their release to various water sources or accumulation in soil that may potentially threaten and endanger human life and the ecosystem (Yusuf 2007). The heavy metals are the potentially toxic contaminants of the runoff sediments (Zhang et al. 2013). These metals may be transported in the road runoff either in dissolved (< 0.45 μm) or in particulate (> 0.45 μm) state (Davis and Birch 2010). The determination of the total concentration of the metals in the runoff and the runoff sediments is a useful indicator, but this does not give complete information about the mobility and availability of the metals (Larner et al. 2008).
The hazards paused by the metals to humans and the ecosystem as a whole could not be ascertained with any degree of certainty if the chemical phases with which the metals are associated are not identified. The different chemical forms of the metals in the sediments exhibit different physical and chemical behaviour in terms of chemical interactions, mobility, bioavailability and potential toxicity (Li et al. 2000). The mobility and reactivity of heavy metals in water and soil can affect their bioavailability, toxicity and distribution in the environment (Xue and Yong 2007). The solubility of the metals in the soil during precipitation and their redistribution into water can further alleviate the situation and will work against the processes of immobilisation through adsorption or complexation (Misra and Chaturvedi 2007). The solubility and bioavailability of the metals are related to the associated chemical phases and are found to decrease in the order, exchangeable > carbonate-bound > Fe–Mn oxide-bound > organic bound > residual (Li et al. 2009). Fractionation of heavy metal cations into operationally defined forms by sequential extraction procedure is an important approach for distinguishing various species of trace elements according to their physicochemical mobility and potential bioavailability (Table 1) (Violante et al. 2010). Studies on the distribution and speciation of heavy metals in sediments can provide information on the degree of pollution as well as the actual environmental impact of the metals based on their bioavailability as well as their origin (Turkey 2007). The metals present in the exchangeable (F1) and carbonate bound (F2) fractions are considered to be weakly bound and may equilibrate with the aqueous phase and becoming more bioavailable (Baruah et al. 1996). Metals in this fraction are the most mobile and readily available for biological uptake in the environment.
The reducible phase (F3) extracts metals from the aqueous phase by the mechanism of adsorption and coprecipitation (Bordas and Bourg 2001). Fe and Mn make up considerable proportion of rocks and sediments; thus the reducible fractions show higher Fe and Mn concentrations.
In case of the oxidisable phase (F4) the affinity of heavy metals for organic substances and their decomposition products are of great importance for the release of the metals into water. Organic matter, with high molecular weight acids, plays an important role in the distribution and dispersion of heavy metals, by mechanisms of chelation and cation exchange processes (Purushothaman and Chakrapani 2007).
The metals associated with crystal structures (F5) are not released easily by mild acids. However, when the samples are digested completely with strong acids, all the metals get into the dissolved forms. Hence, under normal sediment–water interaction, there is not much possibility for the release of these metals. The concentration of heavy metals in the crystalline fraction is largely controlled by the mineralogy and the extent of weathering. Heavy metals in this form are not soluble under experimental conditions and hence may be considered to be held within the mineral matrix (Purushothaman and Chakrapani 2007).
In the present work, sediments collected from road runoff in the city of Guwahati, India, were analysed with respect to four of the common toxic metals, Cd, Co, Cu and Zn, and their relative amounts carried by different chemical phases were determined with a sequential extraction procedure. This helps to obtain a thorough knowledge on distribution, mobility and bioavailability of the metals (Degryse et al. 2003).
Materials and methods
Study area and sampling sites
Guwahati is the ‘Gateway of the entire north-eastern India’, with its location given by 26°10′N and 92°49′E. As per 2011 census, the population of Guwahati is 963,429. The city is situated on an undulating plain with varying altitudes of 49.5–55.5 m above mean sea level and structurally situated on the 50 m thick alluvium of the middle Brahmaputra valley. The central part of the city is interspersed with small hillocks like Sarania Hill (193 m), Nabagraha Hill (217 m), Nilachal hill (193 m) and Chunsali Hill (293 m). The climate of the city is nearly homogenous over the city and is characterized by a cold and foggy winter (December–February), a moderately cool spring (March–May), a fairly hot and humid summer (June–August) and a pleasant autumn (September–November). The average annual rainfall is 180 cm during the months of May–September.
The city is the main corridor for passage to the Indian states of Assam, Meghalaya, Mizoram, Tripura, Manipur, Nagaland and Arunachal Pradesh and all the roads, including the two highways, NH31 and NH37, passing through the city, are busy with vehicular traffic day and night. The sites selected for collection of rainwater runoff in this study (Table 2) were:
-
1.
Jalukbari (J) located in the western part of the city is considered as a major traffic junction for vehicles coming to and going from Guwahati to northern, eastern and western parts of the state including interstate traffic. This site can be described as an institutional and traffic-related site, with Gauhati University campus within the site. A considerable amount of construction activities, including making a bypass of the National Highway NH37 with several overbridges, widening of the road and the bridge over the Brahmaputra have been going on for quite some time.
-
2.
Noonmati (N) growing rapidly around the Indian Oil Corporation Refinery is both industrial and residential in nature, but is mainly categorised as an industrial site. It is also a traffic hub connecting the city to the eastern parts of greater Guwahati as well as the district of Morigaon.
-
3.
Khanapara (K) is one of the main traffic junctions of the city, receiving thoroughfare from upper Assam districts and the state of Meghalaya moving. Although the area is known for several educational institutions like the Veterinary College campus of the Assam Agriculture University, Government offices, business establishments and tourist resorts, the location is mainly a traffic-related site.
-
4.
Maligaon, (M) is a hub of business and traffic activities, and all thoroughfares to the west of the city as well as to the north bank of the River Brahmaputra, cross through this location. The site houses the headquarters of the Northeast Frontier Railway and has expanded tremendously in recent times with business and commerce. This site is also considered as traffic-congested site mainly.
-
5.
Rajgarh (R) is mainly a residential area with a considerable amount of traffic.
-
6.
Ujanbazar (U), near the Brahmaputra River and surrounded on one side by hills, is principally a residential site. The area is one of the oldest settlements of Guwahati and has moderate amount of traffic movement.
-
7.
Kahilipara (Ka) is situated at the south-eastern part of the city with relatively lesser traffic. It is mainly a residential area.
-
8.
Chandmari (C) is considered as the eastern hub of the city, with high traffic at the intersection of two main roads of the city, R G Barooah Road and G N Bardoloi Road. Large and small business activities have grown rapidly on both sides of the G N Bardoloi Road and the area sees almost continuous traffic jam from morning to late evening. The site is considered as a traffic-related site.
-
9.
Zoo Road Tiniali (Z) not far from Chandmari area is busy with considerable traffic along the R G Baruah Road, but on both sides of the road, prime residential areas have grown up. The site is considered mainly as a residential site.
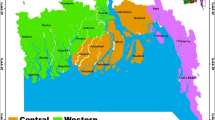
Figure 1 shows the approximate locations of the sampling sites.
Collection of samples and analysis procedures
The road runoff was collected at the nine locations in a few major rain events during and immediately afterwards in two rainy seasons. The sediments were separated from the liquid phase by filtering with Whatmann 42 filter paper. The sediments collected from several rain events in a single location were mixed thoroughly to get a composite, representative sample. These were dried overnight at 70 °C in an air oven and were ground in a manual mortar and were sieved with a 230 mesh sieve to obtain particles of relatively uniform size.
For the speciation of metals, the sequential extraction procedure (Tessier 1979; Yusuf 2007; Mahanta and Bhattacharyya 2011) was followed and five extracts were obtained as follows: 1 g of the runoff sediment was extracted with 10 ml of 1.0 M MgCl2, pH 7.0, 1 h shaking at 300 K to get the exchangeable fraction (F1). The residue from ‘F1’ was treated with 10 ml 1.0 M NaOAc, pH 5.0, 5 h shaking at 300 K to obtain the carbonate-bound phase (F2). The fraction bound to the reducible phase (iron and manganese oxides) (F3) was obtained from the residue of ‘F2’ by treating with 20 ml of 0.04 M NH2OH.HCI in 25% acetic acid at 369 K, 6 h shaking. The residue from ‘F3’ was mixed with 3.0 ml of 0.02 M HNO3 and 5.0 ml of 30% (v/v) H2O2, pH 2.0, heated at 358 K, 2 h shaking, cooled to room temperature, treated with 3.0 ml of 30% (v/v) H2O2, pH 2.0, 3 h shaking at 358 K, cooled and 5.0 ml of 3.2 M NH4OAc in 20% (v/v) HNO3 was added. The solution was diluted to 20 ml, agitated for 30 min. This procedure yielded the fraction (F4) of trace metals bound to organic matter. The residual or lattice metals (F5) was obtained from the residue of ‘F4’ by digesting with tri acid mixture of HCl, HNO3 and H2SO4 in the ratio 1:2:4.
The metals were analysed for each of the extracts with Atomic Absorption Spectrometer (PerkinElmer AAnalyst 200) using three standards calibration method.
Results and discussion
The distribution and mobility of the metals, Cd, Co, Cr, Cu, Mn and Zn, in the runoff sediments, could be discussed on the basis of their contents in the five different fractions. The total concentration of the metals determined separately in all the locations (Fig. 2) was compared with the sum of the contents of the metals in the five fractions. The two sets of values mostly tallied, but differences were also shown in a few cases.
Total concentration (mg/kg) of Cd, Co, Cr, Cu, Mn and Zn in the runoff sediments (Cd is shown with a multiplication factor of 10) (J, N, K, M, R, U, Ka, C and Z, respectively, represent the sampling sites as defined in Table 2)
The ranges and the mean values of the metals are given in Table 3. The statistical summary, viz., the minimum, maximum and mean values of the metals in the five fractions of all the locations are given in Table 4. The following general observations can be made with respect to each metal:
Cadmium
Total Cd contents of the runoff sediments and the sum of Cd in the five fractions differed little except for the location U (Fig. 3a). This might be due to comparatively higher contents of Cd in the runoff sediments of the location U that might be lost partially in the extraction procedure.
a Variation in sum (of the fractions) and total concentration of Cd in sediments of nine runoff locations (top), b concentration of Cd in the five fractions of the runoff sediments (middle), c comparison of the average concentrations of Cd in the five fractions of the runoff sediments (bottom) (J, N, K, M, R, U, Ka, C and Z, respectively, represent the sampling sites as defined in Table 2)
In the sequential extraction (Fig. 3b), Cd was detected in the first three fractions, i.e. exchangeable (F1), carbonate bound (F2) and reducible (F3). The exchangeable fraction (F1) was dominant in the location C, whereas the carbonate fraction (F2) and the fraction bound to the reducible phase (F3) were in abundance at the location U.
From the average values of Cd in all the locations, it was observed that the carbonate bound fraction (F2) of Cd was the dominant fraction (Fig. 3c) while there was negligible Cd presence in the exchangeable fraction (F1). Cd could not be detected in the oxidisable or organically bound fraction (F4) and the residual (F5) fraction. Cd contents followed the trend F2 (48%) > F3 (27%) > F1 (25%) in the three extracts where it was detected. Thus, despite Cd-content being very low, it appeared in the mobile fractions and may easily pass on to water. Lu et al. (2007) proposed the Cd to be the most mobile in his work on the fractionation of urban soil.
Very low solubility of Cd and its inability to form complexes with organic matter might be responsible for very low or below detectable quantity of Cd in the runoff sediments. This was also observed by Sposito et al. (1982). In several earlier works, Cd was found to be the most bioavailable and mobile metal in the study of soil in France (Waterlot et al. 2013), Barcelona (Perez et al. 2008), Bangladesh (Kashem et al. 2007) and Saudi Arabia (Turki 2007) indicating that Cd can be released easily into water and the food chain.
Olajire et al. (2003) and Kashem et al. (2007) have shown that Cd fractions bound to other than the residual can be taken up easily by plants and, therefore, plants growing in the area of higher Cd-availability due to runoff sediment deposition may accumulate Cd in them. Thus, Cd bound to other than the residual fraction may be bioavailable easily.
In fraction F1, the minimum and the maximum values of Cd were found at the locations, Ka and C, respectively. Cd was very low in F2 fraction of the locations, J and R, and high in the location U. Cd in F3 fraction was very low in the locations R and Z, but was comparatively high in the location U.
Similar values of Cd were found but with highest availability in the residual fraction by Mahanta and Bhattacharyya (2011). Jalali and Arfania (2011) have reported that Cd accumulated in 0–2 cm top soil layer. The runoff sediments, which were most likely to have come from the unstable top layers in the city’s soil, however, did not show much Cd in the present work.
Cobalt
The variations in total Co concentration and the sum of the five fractions are shown in Fig. 4a. The two sets of values had some differences, the highest being in the locations, J and K, relative to the other locations. The total Co content varied from 21.6 to 53.27 mg/kg. The highest and the lowest Co contents in the runoff sediments were found in the locations, J and Ka.
a Variation in sum (of the fractions) and total concentration of Co in the sediments of nine runoff locations (top), b concentrations of Co in the five fractions of the runoff sediments (middle), c comparison of the average concentrations of Co in the five fractions of the runoff sediments (bottom) (J, N, K, M, R, U, Ka, C and Z, respectively represent the sampling sites as defined in Table 2)
The distribution of Co in the nine locations is shown in Fig. 4b. The exchangeable fraction (F1) was predominant in the location M; the locations J, N, R, C and Z had almost equal availability; the locations K, U and Ka had very similar contents, but lower than those of the other locations. The carbonate bound fraction (F2) was detected in larger amount in the location J and a similar, slightly lower content was found in the locations, N and U. Co in this fraction was equally predominant in the remaining locations, K, M, R, Ka, C and Z. The third, i.e. the reducible fraction (F3) contained Co almost in the same order in all the locations, yet the values were slightly higher in the locations J, N, M and C; and the location U had the lowest Co content in this fraction. Co in the oxidisable and sulphide bound fraction (F4) was highly predominant in location U followed by almost equal abundance in the other locations. Co was distributed in the reducible fraction (F5) almost equally in all the runoff sediments.
Figure 4c represents the distribution of average concentration of Co in the five fractions. It shows that the five chemical fractions were in the order of F4 (34%) > F1 (30%) > F2 (18%) > F5 (10%) > F3 (9%), i.e. organic and sulphide bound fraction (F4) was predominant among all the fractions. Considerable amounts of Co were found in the exchangeable fractions (F1). This fraction is very labile and can be bioavailable easily (Li et al. 2009). The reducible fraction, F3, was the least available form of Co in the sediments.
It is to be noted that the three fractions, F1, F2 and F3, contained as much as 57% of Co in the sediments that indicated that more than half of Co in the runoff sediments is labile and under suitable environment, may be released to water. Similar trends were reported in earlier researches (Pardo et al. 1990; Braungardt et al. 2007).
Chromium
The variations of total Cr and the sum of the five fractions of the sequential extraction are shown in Fig. 5a. There were considerable differences in the total and the sum of fractions in the runoff sediments of the locations, N and K.
a Variation of total Cr and sum of the five fractions in the runoff sediments (top), b Spatial distribution of Cr in the five fractions (middle), c Comparison of the average concentrations of Cr in the five fractions of the runoff sediments (bottom) (J, N, K, M, R, U, Ka, C and Z respectively represent the sampling sites as defined in Table 2)
Figure 5b shows the Cr content in the sequential extraction procedure spatially. The spatial distribution reveals that the location U had the highest content of Cr in all the fractions (except F2). Cr was predominantly present in the residual fraction, F5 (mean 45%) (Fig. 5c). It means that nearly half of Cr is immobile and is not bioavailable. Similar results were found by Jain et al. 2007 in the sediments of Uttaranchal, India. In an earlier work, Mahanta and Bhattacharyya (2011) have shown that the residual fraction bound Cr was 34% in the street dusts of Guwahati.
The exchangeable fraction (F1) had the lowest Cr in the location Ka and highest in the location U. Location C had the lowest and location M had the highest content in the carbonate bound fraction (F2) of the sediment. The reducible fraction (F3) had the lowest value in the location R and the highest value in the location U. The fraction bound to organic matter (F4) could be detected lowest in the location J and highest in the location U and last the residual fraction (F5) was lowest in the location Ka and highest in the location U. The sequential extraction process revealed that Cr is bound to the different fractions in the following way:
It is clear that only 26% of Cr was found in the first three fractions, F1, F2 and F3 that mostly define mobility of a metal, Therefore, Cr in the runoff sediments had only moderate mobility and about one-fourth of the total Cr may be bioavailable. The F2 fraction was the least predominant fraction of Cr. This may be due to the inability of Cr3+ to form a precipitate or complex with carbonates (Huang et al. 2013).
Copper
Total Cu and the sum of the five fractions of the sequential extraction vary spatially as shown in Fig. 6a. The two sets vary little except at the locations, U and Z. The spatial distribution of Cu in the sequential extraction (Fig. 6b) shows that the exchangeable fraction (F1) was almost equally distributed in all the nine locations. The location Z had the highest value of Cu in exchangeable (F1), carbonate bound (F2) and residual fraction (F5). The location J had Cu mostly in the reducible (F3) and oxidisable (F4) fractions.
a Variation in sum (of the five fractions) and total concentration of Cu in the runoff sediments (top), b concentrations of Cu in the five fractions of the runoff sediments (middle), c comparison of the average concentrations of Cu in the five fractions of the runoff sediments (bottom) (J, N, K, M, R, U, Ka, C and Z respectively represent the sampling sites as defined in Table 2)
The average distribution of Cu (Fig. 6c) in the speciated fractions was
i.e. most copper could be found in the oxidisable (organically bound + sulphide bound) fraction, F4 as in the case of Co. Similar results were earlier found by other workers (Imperato et al. 2003; Jain et al. 2007; Turki 2007, Mahanta and Bhattacharyya 2011; Huang et al. 2013) and the same is attributed to the more pronounced tendency of Cu to form complexes with organic compounds. It is also likely that the stability of organo-Cu complex influenced the affinity of Cu towards humic organic matter (Wang and Qin 2007; Morillo et al. 2004). Zuo et al. 2012) found Cu-organic fraction to be 17.61% in road sediments.
Cu bound to the exchangeable fraction was the least available and this means that Cu in the runoff sediments could not be easily exchanged. It is further observed that the first three fractions F1, F2 and F3 possessed a total of 41% of Cu in the sediments which indicates that nearly half of Cu present in the runoff sediments might be mobile depending on the environmental conditions.
The high levels of F4 coincided with the continuous accumulation of organic matter from large amounts of domestic sewage. The strong positive correlation between organic matter and F4 suggests that copper in the sediments was originally associated with organic matter at the time of deposition (Saad and Badr 2003).
Manganese
Total Mn in the sediments and the sum of Mn in the five fractions of sequential extraction tallied very well except in the locations M, C and Z (Fig. 7a). The spatial distribution of Mn in the five fractions (Fig. 7b) shows that the location U had Mn in highest abundance at all the fractions (except F2).
a Variation of total Mn and sum of the five fractions in the runoff sediments (top), b spatial distribution of Mn in the five fractions of the runoff sediments (middle), c comparison of the average concentrations of Mn in the five fractions of the runoff sediments (bottom) (J, N, K, M, R, U, Ka, C and Z respectively represent the sampling sites as defined in Table 2)
The average concentration of Mn in the five fractions is shown in Fig. 7c. The distribution of Mn in the five fractions followed the trend:
Reducible fraction of Mn was the most predominant in the runoff sediments. Similar result was also detected in the work of Huang et al. (2013). The first three fractions, on the average, contained as much as 72% of total Mn contents making it the most mobile metal in the runoff sediments. Thus, Mn can easily transfer itself from the sediment to the water. Individually studying the fractions, the exchangeable Mn fraction (F1) and organically bound fraction (F4) was detected in very low amount. Such results were found in the study of Zerbe et al. (1999).
In the exchangeable fraction (F1), the location C had the lowest and the location U had the highest Mn contents. The location Z had the lowest and the location K had the highest content of Mn in the carbonate bound fraction (F2). The fraction bound to Fe–Mn oxides (F3) had the minimum content of Mn in the location Ka and the maximum content in the location U. The oxidisable fraction (F4) had the lowest content of Mn in location C and the highest content of Mn in the location U. The location Ka had the lowest and the location U had the highest Mn content in the residual form (F5).
Zinc
The differences between the two sets of the data, total Zn content of the runoff sediments and the sum of Zn in the five fractions were significant only at the locations J, R and C (Fig. 8a). The spatial distribution of Zn in the five fractions of the sequential extraction procedure is shown in Fig. 8b. The exchangeable fraction, F1 had the lowest Zn in the location M and the highest in the location K. The carbonate bound fraction, F2 had the lowest Zn in the location K and the highest in the location U. The reducible fraction, F3 had the lowest and the highest contents in the locations Ka and N, respectively, The oxidisable fraction, F4, had the lowest and highest Zn contents in the locations, Ka and J. The residual fraction, F5, had the lowest and the highest values at the locations, R and J respectively.
a Variation in the sum (of the five fractions) and total concentration of Zn in the runoff sediments (top), b Concentrations of Zn in the five fractions of the runoff sediments (middle), c Comparison of the average concentrations of Zn in the five fractions of the runoff sediments (bottom) (J, N, K, M, R, U, Ka, C and Z respectively represent the sampling sites as defined in Table 2)
The distributions of Zn in the five fractions followed the trend (Fig. 8c):
The residual fraction (F5) is the predominant form of Zn and the exchangeable (F1) was the least abundant in the sediment. The fraction bound to organic matter also contained a large amount of Zn, almost as much as in the F5 fraction. The first three fractions, F1, F2 and F3 possessed, in average, a total of 34% of Zn in the runoff sediments. Thus, Zn in the runoff sediments can be described as moderately labile.
Similar results were reported earlier by Zuo et al. (2012). In the work of Jalali and Arfania (2011), the residual fraction of Zn was the most dominant fraction and the exchangeable fraction was the least dominant in the agricultural soil in Hamadan, western Iran. In the work of Kashem et al. (2007), Zn had the dominance in the residual fraction and was comparatively less mobile. The work of Turki (2007) showed that Zn extraction was least dominant in exchangeable and carbonate bound fractions and, therefore, it was comparatively less mobile and fell in the low- to medium-risk category for the aquatic environment. Canuto et al. (2013) in the work in mobility of trace metals, Zn was detected in residual fraction followed by oxidisable fraction.
Risk assessment
The health risk of the metals can be analysed by using the Risk Assessment Code, RAC (Jain et al. 2007). The risk assessment code can be calculated for the metals to obtain the health risk of the metals to the aquatic bodies or the other living organisms.
As given in Table 5, the sediments which release less than 1% of the total metal in the exchangeable and carbonate fractions are considered safe for the environment. On the contrary, the sediments releasing more than 50% of the total metal in exchangeable and carbonate fractions are considered highly dangerous and metals from such sediments can readily enter the food chain (Perin et al. 1985).
On this basis, the toxicity risks of the metals in the runoff sediments of this work can be summarised as (1) Cd in the sediments is of very high risk as 73% of it was found in the Fractions, F1 and F2, (2) 48% Co appears in the fractions, F1 and F2 and, therefore, fall in the high-risk region, (3) The metals, Cu, Cr, Mn and Zn were present in the F1 and F2 fractions in 18, 16, 23 and 15%, respectively, of the total of the five fractions and, therefore, with respect to each of these metals, the runoff sediments can be put in the medium-risk category.
Conclusion
The present work has yielded a database on the different geo-chemical forms of the metals in the stormwater runoff sediments of Guwahati and their mobility characteristics with the risk assessment studies.
Cd was found to be the most mobile and can enter into the food chain very easily and hence the most bioavailable metal in the runoff sediment. Therefore, Cd though of low concentration is with a very high risk. Co is also highly mobile under proper conditions with high risk to the environment. Cr showed its presence predominantly in the residual fraction (F5) (mean 45%) indicating nearly half of Cr is immobile and is not bioavailable. Cu with its low mobility is not very harmful to the environment. 72% of total Mn contents was mobile and could be bioavailable easily. But Mn was not in high risk for the environment according to the risk assessment study. Zn was the least mobile and the risk assessment factor shows that among the metals, Zn has the medium risk to the environment.
References
Baruah NK, Kotoky P, Bhattacharyya KG, Borah GC (1996) Metal speciation in Jhanji River sediments. Sci Total Environ 193:1–12
Bordas F, Bourg A (2001) Effect of solid/liquid ratio on the remobilization of Cu, Pb, Cd and Zn from polluted river sediment modelling of the results obtained and determination of association constants between the metals and the sediment. Water Air Soil Pollut 128:391–400
Braungardt CB, Achterberg EP, Gledhill M, Nimmo M, Poulichet FE, Cruzado A, Velasquez Z (2007) Chemical speciation of dissolved Cu, Ni, and Co in a contaminated estuary in southwest spain and its influence on plankton communities. Environ Sci Technol 41:4214–4220
Canuto FAB, Garcia CAB, Alves JPH, Passos EA (2013) Mobility and ecological risk assessment of trace metals in polluted estuarine sediments using a sequential extraction scheme. Environ Monit Assess 185:6173–6185
Davis B, Birch G (2010) Comparison of heavy metal loads in stormwater runoff from major and minor urban roads using pollutant yield rating curves. Environ Pollut 158:2541–2545
Degryse P, Muchez P, Six S, Waelkens M (2003) Identification of ore extraction and metal working in ancient times: a case study of Sagalassos (SW Turkey). J Geochem Explor 77(1):65–80
Huang X, Zhu Y, Ji H (2013) Distribution, speciation, and risk assessment of selected metals in the gold and iron mine soils of the catchment area of Miyun Reservoir, Beijing, China. Environ Monit Assess 185:8525–8545
Imperato M, Adamo P, Naimo D, Arienzo M, Stanzione D, Violante P (2003) Spatial distribution of heavy metals in urban soils of Naples city (Italy). Environ Pollut 124:247–256
Jain CK, Malik DS, Yadav R (2007) Metal fractionation study on bed sediments of Lake Nainital, Uttaranchal, India. Environ Monit Assess 130:129–139
Jalali M, Arfania H (2011) Distribution and fractionation of cadmium, copper, lead, nickel, and zinc in a calcareous sandy soil receiving municipal solid waste. Environ Monit Assess 173:241–250. https://doi.org/10.1007/s10661-010-1384-9
Kashem MA, Singh BR, Kawai S (2007) Mobility and distribution of cadmium, nickel and zinc in contaminated soil profiles from Bangladesh. Nutr Cycl Agroecosyst 77:187–198
Larner BL, Palmer AS, Seen AJ, Townsend AT (2008) A comparison of an optimised sequential extraction procedure and dilute acid leaching of elements in anoxic sediments, including the effects of oxidation on sediment metal partitioning. Anal Chim Acta 608:147–157
Li X, Shen Z, Wai OWH, Li YS (2000) Chemical partitioning of heavy metal contaminants in sediments of the Pearl River Estuary. Chem Speciat Bioavailab 12(1):17–25
Li F, Fan Z, Xiao P, Oh K, Ma X, Hou W (2009) Contamination, chemical speciation and vertical distribution of heavy metals in soils of an old and large industrial zone in Northeast China. Environ Geol 57:1815–1823
Lu Y, Zhu F, Chen J, Gan H, Guo H (2007) Chemical fractionation of heavy metals in urban soils of Guangzhou, China. Environ Monit Assess 134:429–439
Mahanta MJ, Bhattacharyya KG (2011) Total concentrations, fractionation and mobility of heavy metals in soils of urban area of Guwahati, India. Environ Monit Assess 173:221–240
Misra V, Chaturvedi PK (2007) Plant uptake/bioavailability of heavy metals from the contaminated soil after treatment with humus soil and hydroxyapatite. Environ Monit Assess 133:169–176
Morillo J, Usero J, Garcia I (2004) Heavy metals distribution in marine sediments from the southwest coast of Spain. Chemosphere 55:431–442
Olajire AA, Ayodele ET, Oyedirdan GO, Oluyemi EA (2003) Levels and speciation of heavy metals in soils of industrial southern Nigeria. Environ Monit Assess 85:135–155
Pardo R, Rarrado E, Lourdes P, Vega M (1990) Determination and speciation of heavy metals in sediments of the Pisuerga River. Water Res 24(3):373–379
Perez G, Mesas ML, Valiente M (2008) Assessment of heavy metals remobilization by fractionation: comparison of leaching tests applied to roadside sediments. Environ Sci Technol 42:2309–2315
Perin G, Craboledda L, Lucchese M, Cirillo R, Dotta L, Zanette ML, Orio AA (1985) Heavy metal speciation in the sediments of northern adriatic sea—a new approach for environmental toxicity determination. In: Lekkas TD (ed) Heavy metal in the environment, vol 2, pp 454–456
Purushothaman P, Chakrapani GJ (2007) Heavy metals fractionation in Ganga River sediments, India. Environ Monit Assess 132:475–489
Saad MAH, Badr NB (2003) Copper speciation in the sediments of the polluted Eastern Harbor of Alexandria, Egypt. In: Proceedings of the third international conference on environment and health, Chennai, India, pp 417–426
Sposito G, Lund L, Chang A (1982) Trace metal chemistry in arid-zone field soils amended with sewage sludge: I. Fractionation of Ni, Cu, Zn, Cd, and Pb in solid phases. Soil Sci Soc Am J 46:260–264
Tessier A (1979) Sequential extraction procedures for the speciation of particulate trace metals. Anal Chem 51:844–851
Turki AJ (2007) Metal speciation (Cd, Cu, Pb and Zn) in sediments from Al Shabab Lagoon, Jeddah, Saudi Arabia. JKAU Mar Sci 18:191–210
Violante A, Cozzolino V, Perelomov L, Caporale AG, Pigna M (2010) Mobility and bioavailability of heavy metals and metalloids in soil environments. J Soil Sci Plant Nutr 10(3):268–292
Wang XS, Qin Y (2007) Some characteristics of the distribution of heavy metals in urban topsoil of Xuzhou, China. Environ Geochem Health 29:11–19
Waterlot C, Bidar G, Pelfrene A, Roussel H, Fourrier H, Douay F (2013) Contamination, fractionation and availability of metals in urban soils in the vicinity of former lead and zinc smelters, France. Pedosphere 23(2):143–159
Xue SW, Yong Q (2007) Leaching characteristics of heavy metals and as from two urban roadside soils. Environ Monit Assess 132:83–92. https://doi.org/10.1007/s10661-006-9504-2
Yusuf KA (2007) Sequential extraction of lead copper, cadmium and zinc in soils near Ojota waste site. J Agron 6(2):331–337
Zerbe J, Sobczyński T, Elbanowska H, Siepak J (1999) Speciation of heavy metals in bottom sediments of lakes. Pol J Environ Stud 8(5):331–339
Zhang J, Deng H, Wang D, Chen Z, Xu S (2013) Toxic heavy metal contamination and risk assessment of street dust in small towns of Shanghai suburban area, China. Environ Sci Pollut Res 20:323–332
Zuo XJ, Fu DF, Li H (2012) Speciation distribution and mass balance of copper and zinc in urban rain, sediments, and road runoff. Environ Sci Pollut Res 19:4042–4048
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Devi, U., Bhattacharyya, K.G. Mobility and bioavailability of Cd, Co, Cr, Cu, Mn and Zn in surface runoff sediments in the urban catchment area of Guwahati, India. Appl Water Sci 8, 18 (2018). https://doi.org/10.1007/s13201-018-0651-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0651-8