Abstract
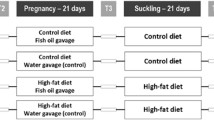
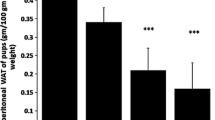
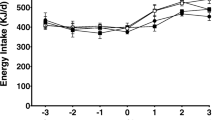
The present study assessed the modulatory potentials of dietary n-3 [α-linolenic acid (ALA, 18:3n-3, eicosapentaenoic acid (EPA, 20:5n-3 + docosahexaenoic acid (DHA) 22:6n-3), and n-6 fatty acid (LA, 18:2n-6)] on anthropometric parameters and fertility indices in high-fat-fed rats. Weanling female Wistar rats were fed with control diet (7% lard), high-fat diet (35% lard, HFL), high-fat with fish oil (21% fish oil + 14% lard, HFF), high-fat with canola oil (21% canola oil + 14% lard, HFC) and high-fat with sunflower oil (21% sunflower oil + 14% lard, HFS) for 2 months, mated and continued on their diets during pregnancy. At gestation day 18–20, the intra-uterine environment was examined in representative rats, and the rest were allowed for delivering pups. The pups after lactation were subjected to mating and feeding trials as above. Growth parameters (body weight, body length (BL), abdominal circumference (AC), thoracic circumference (TC), and Lee index and fertility parameters (litter size and sex ratio) were studied. Feeding HFL diet increased BL (16%), AC (33%) and TC (21%) compared to control (p < 0.05). Adipose tissue accumulation was 11% higher in the HFL group compared to control and was lowered with n-3 fatty acid incorporation in the diet. HFL group exhibited a lower percentage of fertility, pregnancy, and delivery indices. Litter size was decreased by 20%, and litter weight was increased by 23% in HFL group compared to control with more male pups. Our study indicated that n-3 to a larger extent than n-6 fatty acids modulated high-fat induced changes in the anthropometric parameters and fertility indices.


Similar content being viewed by others
References
Acharya P, Talahalli RR (2019) Aging and hyperglycemia intensify dyslipidemia-induced oxidative stress and inflammation in rats: assessment of restorative potentials of ALA and EPA + DHA. Inflammation 42:946–952
Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S (2012) The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol 10:49
Ahmadi A, Akbarzadeh M, Mohammadi F, Akbari M, Jafari B, Tolide-Ie HR (2013) Anthropometric characteristics and dietary pattern of women with polycystic ovary syndrome. Indian J Endocr Metab 17:672
Bernardis L (1970) Prediction of carcass fat, water and lean body mass from Lee’s ‘nutritive ratio’ in rats with hypothalamic obesity. Experientia 26:789–790
Breetha R, Ramaprasad TR (2018) Dietary n-3 but not n-6 fatty acids down-regulate maternal dyslipidemia induced inflammation: a three-generation study in rats. Prostaglandins Leukot Essent Fatty Acids 135:83–91
Bremer AA, Auinger P, Byrd RS (2009) Relationship between insulin resistance–associated metabolic parameters and anthropometric measurements with sugar-sweetened beverage intake and physical activity levels in US adolescents: findings from the 1999–2004. National health and nutrition examination survey. Arch Pediatr Adolesc Med 163:328–335
Campos KE, Volpato GT, Calderon IMP, Rudge MVC, Damasceno DC (2008) Effect of obesity on rat reproduction and on the development of their adult offspring. Braz J Med Biol Res 41:122–125
Cansell M, Nacka F, Combe N, Chessex P, Lavoie J, Rouleau T (2003) Lipid metabolism and therapy. Nutrition 19:275–279
Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF (2008) Maternal high-fat diet and fetal programming increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci 28:12107–12119
Comerford KB, Ayoob KT, Murray RD, Atkinson SA (2016) The role of avocados in maternal diets during the periconceptional period, pregnancy, and lactation. Nutrients 8:313
Devaki G, Shobha R (2018) Maternal anthropometry and low birth weight: a review. Biomed Pharmacol J 11:815–820
Diet CB, Diet U, Diet N (1977) Report of the American Institute of Nutrition ad hoc committee on standards for nutritional studies. J Nutr 107:1340
Du M, Yan X, Tong JF, Zhao J, Zhu MJ (2010) Maternal obesity, inflammation, and fetal skeletal muscle development. Biol Reprod 82:4–12
Fan D, Li L, Li Z, Zhang Y, Ma X, Wu L, Qin G (2018) Effect of hyperlipidemia on the incidence of cardio-cerebrovascular events in patients with type 2 diabetes. Lipids Health Dis 17:102
Fountain ED, Mao J, Whyte JJ, Mueller KE, Ellersieck MR, Will MJ et al (2008) Effects of diets enriched in omega-3 and omega-6 polyunsaturated fatty acids on offspring sex-ratio and maternal behavior in mice. Biol Reprod 78:211–217
Hohos NM, Skaznik-Wikiel ME (2017) High-fat diet and female fertility. Endocrinology 158:2407–2419
Kubagawa HM, Watts JL, Corrigan C, Edmonds JW, Sztul E, Miller MA (2006) Oocyte signals derived from polyunsaturated fatty acids control sperm recruitment in vivo. Nat Cell Biol 8:1143
Ludwig DS, Currie J (2010) The association between pregnancy weight gain and birthweight: a within-family comparison. The Lancet 376:984–990
Mandy M, Nyirenda M (2018) Developmental origins of health and disease: the relevance to developing nations. Int Health 10:66–70
McKeegan PJ, Sturmey RG (2011) The role of fatty acids in oocyte and early embryo development. Reprod Fertil Dev 24:59–67
Moghaddam TF, Saraswathi G (2012) Maternal anthropometric measurements and other factors: relation with birth weight of neonates. Nutr Res Pract 6:132–137
Novelli E, Diniz Y, Galhardi C, Ebaid G, Rodrigues H, Mani F et al (2007) Anthropometrical parameters and markers of obesity in rats. Lab Anim 41:111–119
Ornoy A (2011) Prenatal origin of obesity and their complications: gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol 32:205–212
Ramaiyan B, Bettadahalli S, Talahalli RR (2016) Dietary omega-3 but not omega-6 fatty acids down-regulate maternal dyslipidemia induced oxidative stress: a three generation study in rats. Biochem Biophys Res Commun 477:887–894
Rao KR, Padmavathi I, Raghunath M (2012) Maternal micronutrient restriction programs the body adiposity, adipocyte function and lipid metabolism in offspring: a review. Rev Endocr Metab Disord 13:103–108
Romaguera D, Ängquist L, Du H, Jakobsen MU, Forouhi NG, Halkjær J, Feskens EJ, Masala G, Steffen A, Palli D, Wareham NJ (2011) Food composition of the diet in relation to changes in waist circumference adjusted for body mass index. PLoS ONE 6:23384
Rosenfeld CS, Roberts RM (2004) Maternal diet and other factors affecting offspring sex ratio: a review. Biol Reprod 71:1063–1070
Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH et al (2008) Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51:383–392
Schaefer-Graf UM, Graf K, Kulbacka I, Kjos SL, Dudenhausen J, Vetter K, Herrera E (2008) Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 31:1858–1863
Schmitz G, Ecker J (2008) The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res 47:147–155
Simopoulos AP (2011) Evolutionary aspects of diet: the omega-6/omega-3 ratio and the brain. Mol Neurobiol 44:203–215
Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A et al (2006) The infant development, environment, and lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics 118:1149–1156
Sun B, Purcell RH, Terrillion CE, Yan J, Moran TH, Tamashiro KL (2012) Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes 61:2833–2841
Tellechea ML, Mensegue MF, Pirola CJ (2017) The association between high fat diet around gestation and metabolic syndrome-related phenotypes in rats: a systematic review and meta-analysis. Sci Rep 7:5086
Wathes DC, Abayasekara DRE, Aitken RJ (2007) Polyunsaturated fatty acids in male and female reproduction. Biol Reprod 77:190–201
Woods SC, Seeley RJ, Porte D, Schwartz MW (1998) Signals that regulate food intake and energy homeostasis. Science 280:1378–1383
Acknowledgements
Ms. Breetha acknowledges UGC, New Delhi, for the award of Research Fellowship. The authors thank the Council of Scientific and Industrial Research New Delhi and Director, CSIR-Central Food Technological Research Institute, Mysuru, for all the encouragement. A part of this work was financially assisted by BSC 0404. The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramaiyan, B., Zarei, M., Acharya, P. et al. Dietary n-3 but not n-6 fatty acids modulate anthropometry and fertility indices in high-fat diet fed rats: a two-generation study. J Food Sci Technol 58, 349–355 (2021). https://doi.org/10.1007/s13197-020-04548-6
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04548-6