Abstract
In the study the effect of drying temperature on phenolic and organic acid content, total phenolic content, ergosterol content, antioxidant activity and content of 40 elements in fruiting bodies of Leccinum scabrum and Hericium erinaceus was estimated. The analysis was performed for fresh fruiting bodies and those dried at 20, 40 and 70 °C. Drying resulted in changes in the profile of phenolic and organic acids. Drying generally resulted in losses of the content of total phenolics, ergosterol and antioxidant activity in both species. However, a reduction and an increase of phenolic acids and organic acids were observed. The greatest reduction of the compounds was generally observed at 70 °C. The greatest losses concerned organic acids (some single components and total) (even more than 90% of some compounds). The inhibition of free radicals decreased in the following order: fresh samples > air-dried samples > samples dried at 40 °C > samples dried at 70 °C. The drying temperature affected only selected element contents in fruiting bodies.
Similar content being viewed by others
Introduction
Leccinum scabrum (Bull.) Gray belonging to the Boletaceae family occurs throughout the northern hemisphere and in Australia and New Zealand. It is a wild growing species forming mycorrhiza with birch. Leccinum scabrum is one of the most commonly consumed wild growing mushrooms. The most delicious are the young fruiting bodies and caps. It is suitable for direct consumption, drying or processing. Mushroom shelf life at room temperature after harvest is limited to a few days (Akram and Kwon 2010; Sommer et al. 2010). That is why after the direct harvesting from the natural environment fruiting bodies of L. scabrum are available in trade also as a dried product. The conditions after harvest as well as culinary treatments greatly affect the quality of mushrooms (Lin et al. 2017; Roncero-Ramos et al. 2017; Sudheer et al. 2016). Hericium erinaceus (Bull.) Pers., also called the lion’s mane mushroom, is known as a medicinal mushroom used in East Asia which demonstrates health-promoting effects including antioxidant, antibacterial, anti-aging, anti-tumor and anti-dementia activity and a neuroprotective effect resulting from the presence of more than 80 kinds of bioactive compounds (Friedman 2015; Li et al. 2016; Lu et al. 2014). It is cultivated in many countries in the world.
Fresh fruiting bodies (especially wild growing) are not available all year round, but consumers can choose dried mushrooms instead. Hot-air drying is a relatively cheap, easily controlled and mostly used drying method (Pendre et al. 2012). However the drying can affect content of some bioactive compounds. It is also important for consumers, who are increasingly appreciating mushrooms not only for their taste but also for their nutritional value and healthy properties associated with the presence of biologically active compounds.
The nutritional and health benefits of mushrooms are connected with polysaccharides, proteins and amino acids (e.g. leucine, lysine, methionine, tryptophan), dietary fibre (mainly in the form of chitin), essential unsaturated fatty acids, as well as macro- and micronutrients and vitamins (B1, B2, B12, C, D, niacin, folic acid), phenolics, organic acids, sterols, alkaloids and terpenoids (Anibal et al. 2015; Heleno et al. 2015; Jedidi et al. 2017; Reid et al. 2017; Sułkowska-Ziaja et al. 2012).
Phenolic compounds are a major group of non-nutrient bioactive substances with antioxidant, antibacterial and anti-inflammatory properties which may promote natural defense mechanisms in humans (Chen et al. 2017b; Heleno et al. 2015; Moro et al. 2012; Nowacka et al. 2014; Souilem et al. 2017). Phenolic compounds may modify metabolic and physiological functions of the human organism and exhibit health-promoting effects (Chen et al. 2017b; Moro et al. 2012; Souilem et al. 2017).
Ergosterol (previtamin D2) is a sterol contained in cell membranes which has beneficial effect on human health and many physiological functions due to its antibacterial, anti-inflammatory and anticancer properties, the potential to reduce the incidence of cardiovascular disease as well as inhibition of cyclooxygenase (COX) activity (Chen et al. 2017b; Hu et al. 2006; Phillips et al. 2011; Shao et al. 2010; Zhang et al. 2002). Ergosterol under the influence of radiation is converted to vitamin D2 (a form of vitamin D), essential for human metabolism and found naturally in fungi and plants (Mattila et al. 2002; Phillips et al. 2011; Villares et al. 2014).
Organic acids may prevent various diseases because of their antioxidant properties (citric, malic and succinic acids), antibacterial properties (oxalic acid) and anti-inflammatory properties (formic acid) (Altmeyer et al. 1994; Baati et al. 2011; Kwak et al. 2016; Valentão et al. 2005). The metabolites also prevent browning of mushrooms, extend the shelf life and are also responsible for the taste and aroma of mushrooms (Barros et al. 2013; Brennan et al. 2000; Valentão et al. 2005).
The antioxidant properties of bioactive molecules play an important role in preventing damage caused by free radicals (e.g. age-related disorders and cancer) (Genkinger et al. 2004; Shukla and Singh 2007).
In the study the effect of drying temperature on the content of phenolic and organic acids and antioxidant activity in two different species of mushroom was estimated. Additionally the influence of drying temperature on the content of 40 elements was also estimated. The newly presented data provide some important nutritional information about the differences in fresh and dried mushrooms’ chemical composition.
Materials and methods
Mushroom samples
Wild L. scabrum samples were collected from Wielkopolska Region in Poland (52°40′N 17°10′E) in mid September 2017.
Hericium erinaceus was cultivated on a mixture of beech, birch and oak sawdust (3:1:1 vol) supplemented with wheat bran in the amount 25%, corn flour (1%), sucrose (1%) and CaSO4 (1%) in relation to the substrate dry matter. The mixture was watered to the moisture content of 60%, placed in polypropylene bags (1 kg), closed with a cotton cork and then sterilized (121 °C, 1.5 h). After cooling to room temperature the substrate was inoculated with grain mycelium (20 g per bag) and incubated (25 ± 1 °C and 80–85% air humidity).The cotton cork was removed after the substrate became overgrown with mycelium. Then the bags were placed in the cultivation room (16–18 °C and air humidity 85–90%) and lighted with a fluorescent lamp (day-light) for 12 h per day (200 lx intensity of irradiation). The fruiting bodies were harvested successively when they reached maturity. The fruiting bodies of both species were divided into 4 analysis groups as follows: fresh fruiting bodies, dried at air temperature (20 °C, 48 h), dried at 40 °C (12 h) and 70 °C (7 h) in an oven.
Extraction
The extraction of phenolic and organic acids was carried out on fresh and dried samples. The fresh samples of both species were homogenized, while the dried samples were ground to a fine powder. The samples were mixed with 80% methanol and shaken for 12 h at room temperature for extraction of phenolic acids. Organic acids were extracted by water in an ultrasound bath at ambient temperature. Then the samples were centrifuged at 3000 rpm, evaporated to dryness at 40 °C with rotary vacuum and stored at − 20 °C before analyses. For analysis the extracts were redissolved in 1 mL of deionized water (for organic acids) and in 80% methanol (phenolics).
Total phenolic content
The content of total phenolics (TP) was determined using the Folin–Ciocalteu assay according to Singleton and Rossi (1965) with some modification. The mixture of the extract (100 µL) and the Folin–Ciocalteu reagent (1 mL diluted with distilled H20; 1:1, v/v) and Na2CO3 (3 mL, 20%) was incubated for 30 min in darkness at room temperature. Next the absorbance was measured at 765 nm. The results were expressed as mg of gallic acid equivalent (GAE) per g of dry weight (DW).
UPLC analysis of phenolic compounds
Phenolic acids and organic acids were identified on an ACQUITY UPLC H-Class System and PDA eλ Detector (Waters Corporation, Milford, MA, USA). For separation of the compounds an Acquity UPLC BEH C18 column (2.1 mm × 150 mm, 1.7 µm, Waters) thermostated at 35 °C was used. The gradient elution was performed with water and acetonitrile (both containing 0.1% formic acid, pH = 2) at a flow rate of 0.4 mL/min. Compounds were identified by comparing retention time of the analyzed peaks with the retention time of standards or by adding a specific amount of the standard to the analyzed samples and a repeated analysis. Detection was carried out in a Waters Photodiode Array Detector at λ = 280 nm and λ = 320 nm using an external standard.
Evaluation of DPPH radical scavenging activity
The scavenging effect against the DPPH˙ radical was carried out according to Dong et al. (2012). 1 mL of extract at concentration of 10 mg/mL was mixed with 2.7 mL of methanolic solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals at concentration of 6 µmol/L. The mixture was incubated in the dark for 60 min. Then the absorbance of the mixture was measured at 517 nm. The ability to scavenge DPPH radicals was expressed as follow:
Evaluation of ABTS radical scavenging activity
The scavenging effect against the ABTS radical was carried out as described earlier (Gąsecka et al. 2015). A mixture of 7 mol/L ABTS (2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) and 2.45 mol/L potassium persulfate solution was kept in darkness overnight to generate radicals. Then 100 µL of methanolic extract was mixed with the ABTS solution. After 10 min, the absorbance was measured at 734 nm. The radical scavenging activity was calculated according to the formula above.
Extraction and determination of ergosterol
The extraction procedure was according to Perkowski et al. (2008) with some modification. The mixture of mushroom samples, pure methanol and 2 M NaOH were irradiated twice in a microwave oven for 15 s, cooled, and mixed with 1 M HCl. Then the mixture was extracted with pentane three times. Analyses were carried out in an ACQUITY UPLC H-Class System coupled with a PDA eλ Detector (Waters Corporation, Milford, MA, USA) using an ACQUITY UPLC HSS T3 C18 column (150 mm × 2.1 mm, particle size 1.8 µm) (Waters, Ireland) and 1.7 m ACQUITY UPLC BEH C18 VanGuard Pre-column (Waters, Ireland). The mobile phase was a mixture of methanol, acetonitrile and water (v:v:v; 85:10:5). The isocratic elution with the flow rate of 0.5 mL/min was applied.
Preparation of samples for analysis of element contents
Both dried and fresh samples were digested with 7 mL of concentrated nitric acid (65% nitric acid, Merck, Darmstadt, Germany) in closed Teflon containers in the microwave digestion system Mars 6 Xpress (CEM, Matthews, USA). Samples were then filtered through paper filters and diluted with water to a final volume of 15.0 mL. Each of the samples was processed in 3 replicates.
Instruments and quality control
The inductively coupled plasma optical emission spectrometer Agilent 5110 ICP-OES (Agilent, USA) was used for determination of 40 elements (Al, Ba, Be, Ca, Cd, Ce, Cr, Cu, Dy, Er, Eu, Fe, Gd, Hg, Ho, Ir, K, La, Lu, Mg, Mn, Na, Nd, Ni, P, Pb, Pr, Rb, Sb, Sc, Se, Sm, Sn, Sr, Tb, Tl, Tm, Y, Yb, Zn) under common conditions (see Online Resource). ICP commercial analytical standards—mixed mono- and multi-elemental (Romil, England)—were used for the calibration. The selected wavelengths and validation parameters were as follows: the detection limits obtained follow 3-sigma criteria at the level of 0.0X mg/kg dry weight (DW); precision and upper range of calibration curves are presented in Table 2. Uncertainty for the complete analytical process (including sample preparation) was at the level of 20%. Traceability was checked using the standard reference materials CRM S-1 = loess soil; CRM NCSDC (73,349) = bush branches and leaves; CRM 2709 = soil; CRM 405 = estuarine sediments; and CRM 667 = estuarine sediments with the recovery acceptable for all the elements (80–120%).
Statistical analysis
The results in tables and in figures were expressed as the mean value of three replicates (n = 3) and the standard deviation. Differences between mean values were evaluated by one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test. Statistical analysis was performed using STATISTICA 13.1.
Results and discussion
Total phenolic content
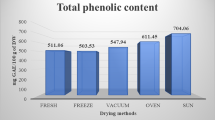
Total phenolic content was nearly two-fold higher in H. erinaceus than in L. scabrum (Fig. 1). TP content in H. erinaceus was lower than in the study presented by Yildiz et al. (2015). The drying method significantly affected TP content in both species. The decrease was confirmed for each drying temperature in comparison to fresh samples. For H. erinaceus it was a 17% reduction of TP (from 3.79 to 3.14 mg/g DW), while for L. scabrum it was a 40% reduction of TP (from 1.89 to 1.14 mg/g DW) at 70 °C in comparison to fresh. The results were in agreement with some studies on mushrooms, fruits and seeds, which contradict the reduction of TP under high-temperature drying (Juhaimi et al. 2018; Sim et al. 2017). The loss of TP (up to 25%) during drying at high-temperature was also confirmed for edible mushroom Phlebopus colossus (Liaotrakoon and Liaotrakoon 2018).
Phenolics are molecules sensitive to temperature, and the changes of the content (both reduction and increase) with different drying temperature was indicated (Jaworska et al. 2014; Jiang et al. 2017; Sim et al. 2017; Yang et al. 2017). The oxygen atmosphere was responsible for their oxidation (Hamrouni-Sellami et al. 2012). In Grifola frondosa mycelia the drying in an oven at 70 °C reduced the TP content only from 17.0 to 16.6 mg GAE/g (Sim et al. 2017). In Lentinula edodes an increase of TP was observed under hot air drying (Yang et al. 2017), because the phenolic compounds are substrates of lignin, whose more intense accumulation was observed as a result of high temperature drying. The reduction of phenolic compounds during drying may be the result of the activation of oxidative enzymes (polyphenol oxidase and peroxidase) (An et al. 2016) and the result of changes in the structure of the phenolic compounds (Toor and Savage 2006). Sezer et al. (2017) did not find any relation between TP and drying temperate of mushrooms. Oven drying at 43 °C did not affect TP or total flavonoid content in Pleurotus ostreatus (Mutukwa et al. 2019). On the other hand, drying at air temperature for 7 days significantly increased TP (from 8.77 to 119.8 mg GAE/g) in the mushroom Amanita zambiana due to the better extractability of bound polyphenols as a result of cell wall destruction after drying (Reid et al. 2017). Jaworska et al. (2014) also found that TP was elevated; however, total flavonoids were lost in Boletus edulis during drying. Šumić et al. (2017) suggested that increase of temperature significantly decreased TP in Cantharellus cibarius, but they predicted that simultaneous use of temperature of 55 °C and vacuum pressure (10 kPa) would preserve phenolic compounds and cause their increase. A study on the effect of drying temperature on TP in jujube fruits showed that phenolics were rather stable at 55 °C and only a small reduction of TP content was observed, but the drying and storage at ambient temperature elevated TP (Pu et al. 2018). In citrus seeds the drying process significantly affected TP content (Juhaimi et al. 2018). The temperatures of 60 and 70 °C elevated or reduced the TP content depending on the citrus seeds, but for all analyzed samples a significant drop was confirmed at 80 °C (Juhaimi et al. 2018).
In the profile of H. erinaceus, caffeic, chlorogenic, ferulic, gallic, 4-hydroxybenzoic, salicylic, sinapic, syringic, t-cinnamic and vanillic acid were quantified (Table 1). Gallic acid was the prevalent phenolic acid in the profile of H. erinaceus (21.5 µg/g DW). In comparison to our earlier study some phenolic compounds were not detected (Gąsecka et al. 2016). To our knowledge, the composition of the phenolic compounds of H. erinaceus has been studied in only a few works. Gallic, ferulic, syringic and vanillic acids were quantified in another study as well (Yildiz et al. 2015), while 4-hydroxybenzoic, ferulic and syringic acids were detected by Li et al. (2012). The drying temperature resulted in the reduction of the content of the acids (from 2 to 37-fold). However, the content of 4-hydroxybenzoic and sinapic acid increased, achieving the highest value at 70 °C. No significant changes in the content of caffeic, ferulic, salicylic, syringic and t-cinnamic acids between different drying temperatures were observed. In the profile of phenolic acids of L. scabrum t-cinnamic and protocatechuic acids were dominant. Additionally, fruiting bodies were characterized by presence of caffeic, chlorogenic, 2,5-dihydroxybenzoic, ferulic, 4-hydroxybenzoic, salicylic, syringic and vanillic acids. Nowacka et al. (2014) also found caffeic, ferulic, 4-hydroxybenzoic and protocatechuic acids in fruiting bodies of L. scabrum. The drying did not affect salicylic acids, but contents of other acids were lower in dried samples. There were no significant changes in the content of ferulic, syringic and vanillic acids between different temperatures of drying. Only air drying had no impact on caffeic, chlorogenic, 2,5-dihydroxybenzoic, 4-hydroxybenzoic and syringic acids. For other acids drying reduced the content. For both species a significant drop of total phenolic acids (sum) was confirmed in drying samples in comparison to fresh samples. Knowledge of changes in the phenolic profile of mushrooms under the drying is very scarce. However, the results obtained for various fruits and vegetables indicated the effect of the drying temperature on the content of phenolic acids. In black rice ferulic, caffeic, p-coumaric and gallic acids were found to be thermally unstable (Lang et al. 2019). Other changes were observed in jujube fruits, because the content of chlorogenic, p-hydroxybenzoic, caffeic, p-coumaric and ferulic acids in dried jujube fruits was significantly higher than in fresh products (Pu et al. 2018). The contents of phenolic compounds of citrus seeds were modified by drying temperate (Juhaimi et al. 2018). Contents of gallic and 3,4 dihydroxybenzoic acids increased at 60 and 70 °C, while the content decreased at 80 °C. The content of caffeic, p-coumaric, trans-ferulic, and syringic acids changed during drying, but both elevation and reduction of the content were observed (Juhaimi et al. 2018). The loss of phenolic compounds may be a result of activation of oxidative enzymes such as polyphenol oxidase and peroxidase (An et al. 2016), and changes in chemical structure of phenolic compounds via binding them to proteins (Toor and Savage 2006). Additionally, in mushrooms during drying, morphological changes may occur in fruiting bodies associated with lignification that affect the phenolic compounds, which are substrates of the lignin (Yang et al. 2017).
Antioxidant activity
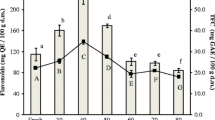
The ability of H. erinaceus and L. scabrum to scavenge DPPH and ABTS radicals is shown in Figs. 2 and 3. Higher potential to scavenge free radicals was exhibited by H. erinaceus. The drying resulted in reduction of radical scavenging ability. The scavenging activity for both species was in the order: fresh samples > air-dried samples > samples dried at 40 °C > samples dried at 70 °C. The inhibition of DPPH radicals decreased from 76 to 53% for H. erinaceus and from 70 to 47% for L. scabrum after drying (mainly from 40 °C in comparison to fresh samples). The inhibition of ABTS radicals decreased from 68 to 45% for H. erinaceus and from 66 to 45% for L. scabrum. The results were in agreement with those of Liaotrakoon and Liaotrakoon (2018), who demonstrated a drastic reduction in the ability to scavenge DPPH radicals during drying of P. colossus. The drying in an oven also significantly reduced DPPH radical scavenging activity of G. frondosa in comparison to fresh mushroom (Sim et al. 2017). Free radical scavenging activity expressed increased from 27.23 to 84.06% for P. ostreatus and from 33.22 to 76.46% for Agaricus bisporus in non-dried samples and those dried at 80 °C (Reid et al. 2017). An increase of antioxidant activity during drying was also observed for B. edulis (Jaworska et al. 2014). The air-dried samples of okra showed lower antioxidant activity in comparison to fresh samples and TP was highly correlated with DPPH-scavenging ability (Juhaimi et al. 2018). However, in citrus seeds the drying process at 60 and 70 °C enhanced antioxidant activity while at 80 °C a decrease was observed (Juhaimi et al. 2018). The ability to scavenge free radicals is connected with phenolic compounds (Šumić et al. 2017). Additionally, Sim et al. (2017) suggested that heating of the mycelium interrupts its ability to donate hydrogen.
Organic acids
Identification of the quantitative analysis of the main organic acids found in fruits, vegetables and mushrooms is considered very important for the assessment of their quality. These acids affect not only the taste, but also their stability, nutrition, acceptability and maintaining quality (Igual et al. 2012; Gao et al. 2012, Colina-Coca et al. 2014, Barros et al. 2013). The beneficial health effects and bioactive properties of some organic acids (e.g. tartaric, malic, citric or succinic acids) are well-known and include antioxidant and antimicrobial, and acidifying properties (Barros et al. 2013; Valentão et al. 2005; Stojković et al. 2017). Metabolites play an important role in mushroom flavour (Ribeiro et al. 2006). Organic acids are more stable during processing and storage than other components such as pigments and flavour compounds (Fernandes et al. 2014). However, information with respect to how their content is affected by various drying methods was, to our knowledge, not available.
The major content of organic acids was observed for fresh mushroom samples, in both studied species (Table 2). Quinic and acetic acids were present in the largest amount in H. erinaceus samples (119.22 and 88.18 µg/g DW), representing 55.78 and 41.26%, respectively, of the total organic acid content. In L. scabrum samples oxalic, succinic and citric acids were dominant (148.19, 34.10 and 28.37 µg/g DW), and accounted for 70.34, 16.19 and 13.47%, respectively. The drying process under different temperatures significantly affected the content of organic acids compared to fresh mushroom samples (Table 2). The content of total analysed organic acids in dried samples of H. erinaceus was ~ 2.4 to 6.9-fold lower (from 213.73 (fresh) to 73.74 and 71.62 for air and 40 °C drying, respectively, while under 70 °C this fall was to 36.96 µg/g DW). In the case of L. scabrum this decrease was definitely higher than that observed in H. erinaceus and ranged from ~ 5.8 to ~ 35-fold (from 210.66 to 31.44, 6.12 and 4.74 µg/g DW under air, 40 °C and 70 °C drying process method, respectively). What should be noted is, that significant decreases were particularly observed for dominant acids obtained in mushroom species samples, even above 95% (e.g. content of quinic acid in H. erinaceus decreased from 119.22 in fresh samples to 0.03 µg/g DW in samples dried under 70 °C and oxalic acid in L. scabrum where the decrease ranged from 148.19 in fresh samples to 0.86 µg/g DW in samples dried under 70 °C. As can be seen, the drying treatments led to a significant decrease (p < 0.05) in the content of major acids and their total sum, which is also connected with the processing method (higher temperature lower content of dominant acid). In H. erinaceus samples, organic acids such as malic, citric, succinic and lactic showed significant differences, associated with their content increase. The content of malic acid increased more than twofold (from 1.39 to 3.33 and 2.87 under air and 40 °C process drying, respectively). The increase in the amount of malic acid extracted from samples treated in the medium dryness process may be caused by activation of its metabolic formation during the glycolytic pathway as a product of the transformation of succinic acid, obtained data for mushrooms correspond with the results described in the literature for other vegetables and fruits (Colina-Coca et al. 2014; Gao et al. 2012). This could be an effect similar to that described by Slatnar et al. (2011). In dried samples of fig fruits, the organic acids were more concentrated, because of lower water content (Slatnar et al. 2011). However, for L. scabrum only a decrease in the content of the studied acids was observed as a consequence of drying under higher temperature, which is likely to be connected with decomposition and degradation of the studied acids (Priecina et al. 2018).
Ergosterol
Ergosterol was detected in all samples, with the highest level in fresh samples (Fig. 4). Differences in ergosterol content were found between fresh and dried samples. All methods of drying reduced the content of the sterol. The strongest effect was confirmed at 70 °C for H. erinaceus and L. scabrum. The drop was about 50 and 48% respectively. Ergosterol is the principal sterol in mushrooms with content related to the species. Losses of the content under drying were detected in some studies (Bernaś 2017; Sławińska et al. 2016), while drying in low temperature indicated that ergosterol content was lower in 15 °C than 5 °C and 10 °C (Vallespir et al. 2019). The studies on A. bisporus confirmed about 30% loss of ergosterol content in air-dried mushrooms in comparison to fresh samples (Bernaś 2017). Additionally, the study revealed that drying method and temperature of storage after drying significantly affected the reduction of the ergosterol level. A decrease of ergosterol content was also found in dried samples of A. bisporus and other species of mushroom—P. ostreatus and L. edodes (Sławińska et al. 2016). Moreover, in the case of hot air dried mushrooms their ability to produce vitamin D after drying under UVB irradiation was confirmed. Storage temperature and UV radiation influence the stability of the ergosterol extract—higher oxidation and production of ergosterol peroxides occurs with increasing temperature, while UV radiation promotes transformation into vitamin D (Villares et al. 2014). Thus the losses of ergosterol in samples of L. scabrum and H. erinaceus were the effect of drying method, the time of storage and access to light.
Element contents
In selected mushrooms the analysis of 40 elements was performed. The obtained results were divided into the following groups of elements: minerals, trace elements, heavy rare earth elements (HREEs) and light rare earth elements (LREEs) (Table 3). The results obtained for minerals (Ca, K, Mg, Na and P) show that the content of elements in fresh fruiting bodies was dependent on the species. The mushroom species richest in Ca, Mg, Na and P was H. erinaceus, while L. scabrum contained a higher content of K. The temperature used during the drying reduced the content of Ca, Mg and P in H. erinaceus, while in L. scabrum Ca, K, Mg, Na and P content decreased after drying. It is difficult to explain why the content of selected elements decreased with an increase in drying temperature. One possible solution could be varied durability of metal complexes with metallothioneins (MTs) being a ubiquitous class of low molecular weight proteins (Muenger and Lerch 1985). Unfortunately, according to our present studies, such an explanation can be real for copper-MTs complexes only. Such complexes can be relatively easy to destroy because of the low durability of numerous MT in higher temperature what confirm analytical studies using a new method for MTs determination (Ryvolova et al. 2011). The results obtained for trace elements (Al, Ba, Be, Cd, Cr, Cu, Fe, Hg, Ir, Mn, Ni, Pb, Rb, Sb, Se, Sn, Sr, Tl, Zn) show that higher contents of them generally are accumulated in fresh fruiting bodies for H. erinaceus. The temperature of drying significantly modified the selected trace element contents similarly to the observation of Garba and Oviosa (2019), who dried Vernonia amygdalina using air, sun, oven and solar drying. The use of different drying methods (different temperatures) caused changes in the content of selected elements (minerals, Fe, Pb, Cu). In spite of slight changes, the decrease in content of these elements was significant (α = 0.05) for Ba, Cd, Cr, Hg, Ir, Mn, Ni, Rb, Se, Sn and Zn. The obtained results show the highest content of Al, Ba, Cu, Hg, Pb, Se and Tl in L. scabrum fresh fruiting bodies and that the increase of temperature of drying was associated with a significant decrease in content of these toxic elements.
For the majority of HREEs, their content in fruit bodies of both species was below detection limits, with the exception of Tm, being in the range 0.03–0.06 mg/kg DW. Among LREEs determined in H. erinaceus, changes in the content of Ce, La, Nd and Pr with a reduction for Ce, Nd and Pr with regard to different temperatures of drying were recorded. No changes were observed for Eu, while Gd and Sm were below the limit of detection. A reduction of La, Nd and Pr for L. scabrum exposed to increased temperature of drying was observed. The other LREEs (Eu, Gd, Nb) were below the limit of detection or no changes were observed.
The obtained results have shown no significant influence of different temperatures of the drying process on content of selected elements in H. erinaceus and L. scabrum fruiting bodies, which confirms the previous data described by Muyanja et al. (2014). In our opinion, changes in element contents (especially at 70 °C) in mushroom bodies are possible mainly for volatile elements such as the herein studied Sb or Se, as was clearly observed, and also As, not analyzed here. In the case of volatile elements their loss is a common occurrence. Therefore before analysis of the above mentioned elements as well as Hg, a subject of numerous studies of mushrooms, the selection of a suitable drying method or the use of a relatively low temperature is necessary (Hojdová et al. 2015). It is worth noting that a higher drying temperature reduces the time of this stage of sample preparation, although it may be associated with the loss not only of volatile elements but also of heavy metals such as Pb or Zn (Zhang et al. 2001).
Changes in element contents may be more associated with the form of elements than with their total concentration, which confirms the study of Chen et al. (2017a), who described the influence of speciation changes of Cd, Cr and Pb after drying (hot air and freeze drying samples). Mushrooms dried in different ways may be characterized by various contents of numerous elements (Maray et al. 2018), whereas this does not apply to mushrooms dried by the same method but with different temperatures.
Conclusion
Drying is a process that allows the product to be stored for a longer time and during the off-season, especially for wild growing mushrooms. Drying is also a method of preservation allowing the product to be available all year round. However, temperature significantly affects the content of the analyzed bioactive compounds and antioxidant properties. Losses of TP, phenolic and organic acids and ergosterol content were observed. Changes in elements were probably connected with speciation changes. The obtained results suggested that losses of bioactive compounds probably diminish the pro-health properties of mushrooms.
References
Akram K, Kwon J-H (2010) Food irradiation for mushrooms: a review. J Korean Soc Appl Biol Chem 53:257–265. https://doi.org/10.3839/jksabc.2010.041
Altmeyer PJ, Mattlies U, Pawlak F, Hoffmann K, Frosch PJ, Ruppert P, Wassilew SW, Horn T, Kreysel HW, Lutz G, Barth J, Rietzschel I, Joshi RK (1994) Antipsoriatic effects of fumaric acid derivatives. Results of a multicenter double-blind study in 100 patients. J Am Acad Dermatol 30:977–981. https://doi.org/10.1016/S0190-9622(94)70121-0
An K, Zhao D, Wang Z, Wu J, Xu Y, Xiao G (2016) Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem 197:1292–1300. https://doi.org/10.1016/j.foodchem.2015.11.033
Anibal C, Farenzena S, Rodríguez MS, Albertengo L (2015) Chemical composition and nutritional value of Argentine commercial edible mushrooms. J Verbrauch Lebensm 10(2):155–164. https://doi.org/10.1007/s00003-015-0937-9
Baati T, Horcajada P, Gref R, Couvreur P, Serre C (2011) Quantification of fumaric acid in liver, spleen and urine by high-performance liquid chromatography coupled to photodiode-array detection. J Pharm Biomed Anal 56(4):758–762. https://doi.org/10.1016/j.jpba.2011.07.011
Barros L, Pereira C, Ferreira ICFR (2013) Optimized analysis of organic acids in edible mushrooms from portugal by ultra fast liquid chromatography and photodiode array detection. Food Anal Methods 6:309–316. https://doi.org/10.1007/s12161-012-9443-1
Bernaś E (2017) Culinary-medicinal mushroom products as a potential source of vitamin D. Int J Med Mushrooms 19(10):925–936. https://doi.org/10.1615/IntJMedMushrooms.2017024596
Brennan M, Le Port G, Gormley R (2000) Post-harvest treatment with citric acid or hydrogen peroxide to extend the shelf life of fresh sliced mushrooms. LWT-Food Sci Technol 33:285–289. https://doi.org/10.1006/fstl.2000.0657
Chen C, Chen G, Wang S, Pei F, Hu Q, Zhao L (2017a) Speciation changes of three toxic elements in Lentinus edodes after drying and soaking. J Food Process Preserv 41(2):e12772. https://doi.org/10.1111/jfpp.12772
Chen S, Tianqiao Y, Zhang Y, Su J, Jiao C, Xie Y (2017b) Anti-tumor and anti-angiogenic ergosterols from Ganoderma lucidum. Front Chem 5:85. https://doi.org/10.3389/fchem.2017.00085
Colina-Coca C, de Ancos B, Sánchez-Moreno C (2014) Nutritional composition of processed onion: s-alk(en)yl-l-cysteine sulfoxides, organic acids, sugars, minerals, and vitamin C. Food Bioprocess Technol 7:289–298. https://doi.org/10.1007/s11947-013-1150-4
Dong J, Zhang M, Lu L, Sun L, Xu M (2012) Nitric oxide fumigation stimulates flavonoid and phenolic accumulation and enhances antioxidant activity of mushroom. Food Chem 135:1220–1225. https://doi.org/10.1016/j.foodchem.2012.05.055
Fernandes A, Barros L, Antonio AL, Barreira JCM, Oliveira MBPP, Martins A, Ferreira ICFR (2014) Using gamma irradiation to attenuate the effects caused by drying or freezing in Macrolepiota procera organic acids and phenolic compounds. Food Bioprocess Technol 7:3012–3021. https://doi.org/10.1007/s11947-013-1248-8
Friedman M (2015) Chemistry, nutrition, and health-promoting properties of Hericium erinaceus (Lion’s Mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J Agric Food Chem 63:7108–7123. https://doi.org/10.1021/acs.jafc.5b02914
Gao Q-H, Wu Ch-S, Wang M, Xu B-N, Du L-J (2012) Effect of drying of jujubes (Ziziphus jujuba Mill.) on the contents of sugars, organic acids, α-tocopherol, β-carotene, and phenolic compounds. J Agric Food Chem 60:9642–9648. https://doi.org/10.1021/jf3026524
Garba ZN, Oviosa S (2019) The effect of different drying methods on the elemental and nutritional composition of Vernonia amygdalina (bitter leaf). J Taibah Univ Sci 13:396–401. https://doi.org/10.1080/16583655.2019.1582148
Gąsecka M, Mleczek M, Siwulski M, Niedzielski P, Kozak L (2015) The effect of selenium on phenolics and flavonoids in selected edible white rot fungi. LWT-Food Sci Technol 63(1):726–731. https://doi.org/10.1016/j.lwt.2015.03.046
Gąsecka M, Mleczek M, Siwulski M, Niedzielski P, Kozak L (2016) Phenolic and flavonoid content in Hericium erinaceus, Ganoderma lucidum and Agrocybe aegerita under selenium addition. Acta Aliment Hung 45(2):301–309. https://doi.org/10.1556/066.2016.45.2.18
Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ (2004) Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol 160(12):1223–1233. https://doi.org/10.1093/aje/kwh339
Hamrouni-Sellami I, Rahali FZ, Rebey IB, Bourgou S, Limam F, Marzouk B (2012) Total phenolics, flavonoids, and antioxidant activity of sage (Salvia officinalis L.) plants as affected by different drying methods. Food Bioprocess Technol 6(3):806–817. https://doi.org/10.1007/s11947-012-0877-7
Heleno SA, Ferreira RC, Antonio AL, Queiroz MJRP, Barros L, Ferreira ICFR (2015) Nutritional value, bioactive compounds and antioxidant properties of three edible mushrooms from Poland. Food Biosci 11:48–55. https://doi.org/10.1016/j.fbio.2015.04.006
Hojdová M, Rohovec J, Chrastný V, Penížek V, Navrátil T (2015) The influence of sample drying procedures on mercury concentrations analyzed in soils. Bull Environ Contam Toxicol 94(5):570–576. https://doi.org/10.1007/s00128-015-1521-9
Hu SH, Liang ZC, Chia YC, Lien JL, Chen KS, Lee MY, Wang JC (2006) Antihyperlipidemic and antioxidant effects of extracts from Pleurotus citrinopileatus. J Agric Food Chem 54:2103–2110
Igual M, García-Martínez E, Martín-Esparza ME, Martínez-Navarrete N (2012) Effect of processing on the drying kinetics and functional value of dried apricot. Food Res Int 47:284–290. https://doi.org/10.1016/j.foodres.2011.07.019
Jaworska G, Pogoń K, Bernaś E, Skrzypczak A (2014) Effect of different drying methods and 24-month storage on water activity, rehydration capacity, and antioxidants in Boletus edulis mushrooms. Dry Technol 32(3):291–300. https://doi.org/10.1080/07373937.2013.824895
Jedidi IK, Ayoub IK, Philippe T, Bouzouita N (2017) Chemical composition and nutritional value of three Tunisian wild edible mushrooms. J Food Meas Charact 11(4):2069–2075. https://doi.org/10.1007/s11694-017-9590-6
Jiang N, Liu C, Li D, Zhang Z, Liu C, Wang D, Niu L, Zhang M (2017) Evaluation of freeze drying combined with microwave vacuum drying for functional okra snacks: antioxidant properties, sensory quality, and energy consumption. LWT-Food Sci Technol 82(1):216–226. https://doi.org/10.1016/j.lwt.2017.04.015
Juhaimi FA, Özcan MM, Uslu N, Ghafoor K (2018) The effect of drying temperatures on antioxidant activity, phenolic compounds, fatty acid composition and tocopherol contents in citrus seed and oils. J Food Sci Technol 55:190. https://doi.org/10.1007/s13197-017-2895-y
Kwak AM, Lee IK, Lee SY, Yun BS, Kang HW (2016) Oxalic Acid from Lentinula edodes culture filtrate: antimicrobial activity on phytopathogenic bacteria and qualitative and quantitative analyses. Mycobiology 44(4):338. https://doi.org/10.5941/MYCO.2016.44.4.338
Lang HG, Lindemann JS, Ferreira DC, Hoffmann JF, Vanier NL, de Oliveira M (2019) Effects of drying temperature and long-term storage conditions on black rice phenolic compounds. Food Chem 287:197–204. https://doi.org/10.1016/j.foodchem.2019.02.028
Li H, Park S, Moon B, Yoo Y, Lee Y, Lee C (2012) Targeted phenolic analysis in Hericium erinaceum and its antioxidant activities. Food Sci Biotechnol 21:881–888. https://doi.org/10.1007/s10068-012-0114-1
Li QZ, Wu D, Zhou S, Liu FY, Li ZP, Feng J, Yang Y (2016) Structure elucidation of a bioactive polysaccharide from fruiting bodies of Hericium erinaceus in different maturation stages. Carbohydr Polym 144:196–204. https://doi.org/10.1016/j.carbpol.2016.02.051
Liaotrakoon W, Liaotrakoon V (2018) Influence of drying process on total phenolics, antioxidative activity and selected physical properties of edible bolete (Phlebopus colossus (R. Heim) Singer) and changes during storage. Food Sci Technol 38(2):231–237. https://doi.org/10.1590/1678-457X.34116
Lin Q, Lu Y, Zhang J, Liu W, Guan W, Wang Z (2017) Effects of high CO2 in-package treatment on flavor, quality and antioxidant activity of button mushroom (Agaricus bisporus) during postharvest storage. Postharvest Biol Technol 123:112–118. https://doi.org/10.1016/j.postharvbio.2016.09.006
Lu QQ, Tian JM, Wei J, Gao JM (2014) Bioactive metabolites from the mycelia of the basidiomycete Hericium erinaceus. Nat Prod Res 28:1288–1292. https://doi.org/10.1080/14786419.2014.898145
Maray AR, Mostafa MK, El-Fakhrany AED (2018) Effect of pretreatments and drying methods on physico-chemical, sensory characteristics and nutritional value of oyster mushroom. J Food Process Preserv 42(1):e13352. https://doi.org/10.1111/jfpp.13352
Mattila P, Lampi AM, Ronkaine R, Toivob J, Piironen V (2002) Sterol and vitamin D2 contents in some wild and cultivated mushrooms. Food Chem 76:293–298. https://doi.org/10.1016/S0308-8146(01)00275-8
Moro C, Palacios I, Lozano M, D’Arrigo M, Guillamón E, Villares A (2012) Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem 130:350–355. https://doi.org/10.1016/j.foodchem.2011.07.049
Muenger K, Lerch K (1985) Copper metallothionein from the fungus Agaricus bisporus: chemical and spectroscopic properties. Biochemistry 24(24):6751–6756. https://doi.org/10.1021/bi00345a004
Mutukwa IB, Hall CA III, Cihacek L, Lee CW (2019) Evaluation of drying method and pretreatment effects on the nutritional and antioxidant properties of oyster mushroom (Pleurotus ostreatus). J Food Process Preserv 43(4):e13910. https://doi.org/10.1111/jfpp.13910
Muyanja C, Kyambadde D, Namugumya B (2014) Effect of pretreatments and drying methods on chemical composition and sensory evaluation of oyster mushroom (Pluerotus oestreatus) powder and soup. J Food Process Preserv 38(1):457–465. https://doi.org/10.1111/j.1745-4549.2012.00794.x
Nowacka N, Nowak R, Drozd M, Olech M, Los R, Malmet A (2014) Analysis of phenolic constituents, antiradical and antimicrobial activity of edible mushrooms growing wild in Poland. LWT-Food Sci Technol 59:689–694. https://doi.org/10.1016/j.lwt.2014.05.041
Pendre NK, Nema PK, Sharma HP, Rathore SS, Kushwah SS (2012) Effect of drying temperature and slice size on quality of dried okra (Abelmoschus esculentus (L.) moench). J Food Sci Technol 49(3):378–381. https://doi.org/10.1007/s13197-011-0427-8
Perkowski J, Buśko M, Stuper K, Kostecki M, Matysiak A, Szwajkowska-Michałek L (2008) Concentration of ergosterol in small-grained contaminated and inoculated cereals. Biologia 63:542–547. https://doi.org/10.2478/s11756-008-0083-2
Phillips KM, Ruggio DM, Horst RL, Minor B, Simon RR, Feeney MJ, Haytowitz DB (2011) Vitamin D and sterol composition of 10 types of mushrooms from retail suppliers in the United States. J Agric Food Chem 59:7841–7853. https://doi.org/10.1021/jf104246z
Priecina L, Karklina D, Kince T (2018) The impact of steam-blanching and dehydration on phenolic, organic acidcomposition, and total carotenoids in celery roots. Innov Food Sci Emerg Technol 49:192–201. https://doi.org/10.1016/j.ifset.2018.01.008
Pu Y, Ding T, Wang W, Xiang Y, Ye X, Lie M, Liua D (2018) Effect of harvest, drying and storage on the bitterness, moisture, sugars, free amino acids and phenolic compounds of jujube fruit (Zizyphus jujuba cv. Junzao). J Sci Food Agric 98:628–634. https://doi.org/10.1002/jsfa.8507
Reid T, Merjury M, Takafira M (2017) Effect of cooking and preservation on nutritional and phytochemical composition of the mushroom Amanita zambian. Food Sci Nutr 5(3):538–544. https://doi.org/10.1002/fsn3.428
Ribeiro B, Rangel J, Valentão P, Baptista P, Seabra RM, Andrade PB (2006) Contents of carboxylic acids and two phenolics and anti-oxidant activity of dried Portuguese wild edible mushrooms. J Agric Food Chem 54:8530–8537. https://doi.org/10.1021/jf061890q
Roncero-Ramos I, Mendiola-Lanao M, Pérez-Clavijo M, Delgado-Andrade C (2017) Effect of different cooking methods on nutritional value and antioxidant activity of cultivated mushrooms. Int J Food Sci Nutr 68(3):287–297. https://doi.org/10.1080/09637486.2016.1244662
Ryvolova M, Krizkova S, Adam V, Beklova M, Trnkova L, Hubalek J, Kizek R (2011) Analytical methods for metallothionein detection. Curr Anal Chem 7:243–261. https://doi.org/10.2174/1573411011107030243
Sezer YC, Süfer Ö, Sezer G (2017) Extraction of phenolic compounds from oven and microwave dried mushrooms (Agaricus bisporus and Pleurotus ostreatus) by using methanol, ethanol and aceton as solvents. Indian J Pharm Educ 51(3s2):s393–s397. https://doi.org/10.5530/ijper.51.3s.55
Shao S, Hernandez M, Kramer JKG, Rinker DL, Tsao R (2010) Ergosterol profiles, fatty acid composition, and antioxidant activities of button mushrooms as affected by tissue part and developmental stage. J Agric Food Chem 58:11616–11625. https://doi.org/10.1021/jf102285b
Shukla Y, Singh M (2007) Cancer preventive properties of ginger: a brief review. Food Chem Toxicol 45(5):683–690. https://doi.org/10.1016/j.fct.2006.11.002
Sim KY, Liew JY, Ding XY, Chddng WS, Intan S (2017) Effect of vacuum and oven drying on the radical scavenging activity and nutritional contents of submerged fermented Maitake (Grifola frondosa) mycelia. Food Sci Technol 37(1):131–135. https://doi.org/10.1590/1678-457X.28816
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult 16:144–158
Slatnar A, Klancar U, Stampar F, Veberic R (2011) Effect of drying of figs (Ficus carica L.) on the contents of sugars, organic acids, and phenolic compounds. J Agric Food Chem 59:11696–11702. https://doi.org/10.1021/jf202707y
Sławińska A, Fornal E, Radzki W, Skrzypczak K, Zalewska-Korona M, Michalak-Majewska M, Parfieniuk E, Stachniuk A (2016) Study on vitamin D2 stability in dried mushrooms during drying and storage. Food Chem 199:203–209. https://doi.org/10.1016/j.foodchem.2015.11.131
Sommer I, Schwartz H, Solar S, Sontag G (2010) Effect of gamma-irradiation on flavour 5′-nucleotides, tyrosine, and phenylalanine in mushrooms (Agaricus bisporus). Food Chem 123(1):171–174. https://doi.org/10.1016/j.foodchem.2010.03.124
Souilem F, Fernandes A, Calhelha RC, Barreira JCM, Barros L, Skhiri F, Martins A, Ferreira ICFR (2017) Wild mushrooms and their mycelia as sources of bioactive compounds: antioxidant, anti-inflammatory and cytotoxic properties. Food Chem 230:40–48. https://doi.org/10.1016/j.foodchem.2017.03.026
Stojković DS, Kovačević-Grujičić N, Reis FS, Davidović S, Barros L, Popović J, Pertrović I, Pavić A, Glamočlija J, Ćirić A, Stevanović M, Ferreira ICFR, Soković M (2017) Chemical composition of the mushroom Meripilus giganteus Karst. and bioactive properties of its methanolic extract. LWT-Food Sci Technol 79:454–462. https://doi.org/10.1016/j.lwt.2017.01.045
Sudheer S, Yeoh WK, Manickam S, Ali A (2016) Effect of ozone gas as an elicitor to enhance the bioactive compounds in Ganoderma lucidum. Postharvest Biol Technol 117:81–88. https://doi.org/10.1016/j.postharvbio.2016.01.014
Sułkowska-Ziaja K, Muszyńska B, Motyl P, Pasko P, Ekiert H (2012) Phenolic compounds and antioxidant activity in some species of polyporoid mushrooms from Poland. Int J Med Mushrooms 14:385–393. https://doi.org/10.1615/IntJMedMushr.v14.i4.60
Šumić Z, Tepić A, Vidović S, Vakula A, Vladić J, Pavlić B (2017) Process optimization of chanterelle (Cantharellus cibarius) mushrooms vacuum drying. J Food Process Preserv 5(5):989–996. https://doi.org/10.1111/jfpp.12822
Toor RK, Savage GP (2006) Effect of semi-drying on the antioxidant components of tomatoes. Food Chem 94(1):90–97. https://doi.org/10.1016/j.foodchem.2004.10.054
Valentão P, Lopes G, Valente M, Barbosa P, Andrade PB, Silva BM, Baptista P, Seabra RM (2005) Quantification of nine organic acids in wild mushrooms. J Agric Food Chem 53:3626–3630
Vallespir F, Crescenzo L, Rodríguez Ó, Marra F, Simal S (2019) Intensification of low-temperature drying of mushroom by means of power ultrasound: effects on drying kinetics and quality parameters. Food Bioprocess Technol 12(5):839–851. https://doi.org/10.1007/s11947-019-02263-5
Villares A, Mateo-Vivaracho L, García-Lafuente A, Eva Guillamón E (2014) Storage temperature and UV-irradiation influence on the ergosterol content in edible mushrooms. Food Chem 147:252–256. https://doi.org/10.1016/j.foodchem.2013.09.144
Yang W, Du H, Mariga AM, Pei F, Ma N, Hu Q (2017) Hot air drying process promotes lignification of Lentinus edodes. LWT-Food Sci Technol 84:726–732. https://doi.org/10.1016/j.lwt.2017.06.039
Yildiz O, Can Z, Laghari AO, Şahin H, Malkoç M (2015) Wild edible mushrooms as a natural source of phenolics and antioxidants. J Food Biochem 39(2):148–154. https://doi.org/10.1111/jfbc.12107
Zhang S, Wang S, Shan X-q (2001) Effect of sample pretreatment upon the metal speciation in sediments by a sequential extraction procedure. Chem Spec Bioavailab 13(3):69–74. https://doi.org/10.3184/095422901782775435
Zhang Y, Mills GL, Nair MG (2002) Cyclooxygenase inhibitory and antioxidant compounds from the mycelia of the edible mushroom Grifola frondosa. J Agric Food Chem 50:7581–7585. https://doi.org/10.1021/jf0257648
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gąsecka, M., Siwulski, M., Magdziak, Z. et al. The effect of drying temperature on bioactive compounds and antioxidant activity of Leccinum scabrum (Bull.) Gray and Hericium erinaceus (Bull.) Pers.. J Food Sci Technol 57, 513–525 (2020). https://doi.org/10.1007/s13197-019-04081-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-04081-1