Abstract
Introduction
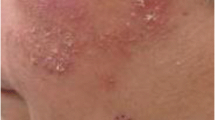
Enzalutamide is a new potent inhibitor of the signaling pathway for the androgen receptor with a half-life of 5.8 days. It has been on the market for the treatment of metastatic castration-resistant prostate cancer since November 2013. We report a case of acute generalized exanthematous maculopapular rash induced by enzalutamide. In summary, newer androgen receptor blockers have a propensity to cause skin related adverse effects. Most common among these are apalutamide. Enzalumatamide, per se, is a safe drug and has not been associated frequently in causing maculopapular rash. Few cases has been reported. In all these cases, the drug was discontinued and 2nd line therapy was instituted. In this report, Enzalutamide was withheld for 10 days and anti-histaminics was instituted. After a full recovery, Enzalutamide was reinstituted in treatment. A 62-year-old male patient with no significant medical history, was diagnosed in March 2020 with metastatic prostatic adenocarcinoma. Baseline PSA was 456 ng/ml. PSMA PET scan showed evidence of multiple bony metastasis. He was started on Degarelix subcutaneous injection with oral abiraterone initially. PSA level showed initial decreasing trend till September 2021 followed by sudden increase. Intramuscular Injection leuprolide was started and initial responses were good followed by later rise of PSA from January. Tab Xtandi (Enzalutamide) was added to the regimen from 31.1.22. Three days after starting enzalutamide treatment, the patient experienced an acute skin reaction. It is about of the plaques covered with widespread millimetric non-follicular papules. Enzalutamide was stopped after appearance of rashes to avoid further serious adverse effects. Anti-histaminics were started. Complete resolution of skin lesions occurred within 10 days. Tab Enzalutamide was reinstituted on 11th day after stoppage and on complete resolution of skin resolutions. According to the CTCAE 5.0 criteria, these skin rash was graded as grade 2. In view of evidence in literature and clinical improvement after stoppage, the acute drug reaction was attributed to enzalutamide. Uro oncologist can be confronted with adverse skin drug reactions attributable to new therapeutic molecules. The slow resolution of symptoms seems be due to the long half-life of enzalutamide. It should not be withdrawn from therapy owing to these effects. Rather, it should be with stopped for 10–14 days. Basic treatment with anti-histaminics or topical steroids may be enough to warranty the resolution of symptoms, and the drug (Enzalutamide) can be continued thereafter.


Similar content being viewed by others
References
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA, Investigators TAX (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351(15):1502–1512. https://doi.org/10.1056/NEJMoa040720
Beer TM, Armstrong AJ, Rathkopf D, Loriot Y, Sternberg CN, Higano CS, Iversen P, Evans CP, Kim CS, Kimura G, Miller K, Saad F, Bjartell AS, Borre M, Mulders P, Tammela TL, Parli T, Sari S, van Os S, Theeuwes A, Tombal B (2017) Enzalutamide in men with chemotherapy-naive metastatic castration-resistant prostate cancer: extended analysis of the phase 3 prevail study. Eur Urol 71(2):151–154. https://doi.org/10.1016/j.eururo.2016.07.032
Hong JH (2018) Pharmacokinetic/pharmacodynamic drug evaluation of enzalutamide for treating prostate cancer. Expert Opin Drug Metab Toxicol 14(3):361–369. https://doi.org/10.1080/17425255.2018.1440288
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, Coskinas X, Frydenberg M, Hague WE, Horvath LG, Joshua AM, Lawrence NJ, Marx G, McCafrey J, McDermott R, McJannett M, North SA, Parnis F, Parulekar W et al (2019) Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. https://doi.org/10.1056/NEJMoa1903835
Hussain M, Fizazi K, Saad F et al (2018) Enzalutamide in men with nonmetastatic, castration- resistant prostate cancer. N Engl J Med 378:2465–2474
Alberto C, Konstantinou MP, Martinage C et al (2016) Enzalutamide induced acute generalized exanthematous pustulosis. J Dermatol Case Rep 10:35–38
Lee S, Chung YJ, Kim BH et al (2009) Comparative pharmacokinetic evaluation of two formulations of bicalutamide 50-mg tablets: an open-label, randomized-sequence, single- dose, two-period crossover study in healthy Korean male volunteers. Clin Ther 31:3000–3008
Chodak G, Sharifi R, Kasimis B et al (1995) Single-agent therapy with bicalutamide: a comparison with medical or surgical castration in the treatment of advanced prostate carcinoma. Urology. 46:849–855
Kolvenbag GJCM, Blackledge GRP (1996) Worldwide activity and safety of bicalutamide: a summary review. Urology 47:70–79
Fizazi K, Shore N, Tammela TL et al (2019) Darolutamide in nonmetastatic, castration- resistant prostate cancer. N Engl J Med 380:1235–1246
Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein JM, Stenzl A (2019) ARCHES: efficacy of androgen deprivation therapy (ADT) with enzalutamide (ENZA) or placebo (PBO) in metastatic hormone-sensitive prostate cancer (mHSPC). J Clin Oncol 37(15_suppl):5048–5048. https://doi.org/10.1200/JCO.2019.37.15_suppl.5048
Saito-Sasaki N, Sawada Y, Okada E, Nakamura M (2018) Drug eruption caused by enzalutamide: a case and literature review of androgen receptor inhibitor related drug eruptions. Australas J Dermatol 59(2):e133–e134. https://doi.org/10.1111/ajd.12694
Alberto C, Konstantinou MP, Martinage C, Casassa E, Tournier E, Bagheri H et al (2016) Enzalutamide induced acute generalized exanthematous pustulosis. Dermatol Case Rep 10(2):35–38. https://doi.org/10.3315/jdcr.2016.1226
Deng M, Chai H, Yang M, Wei X, Zhang W, Wang X, Li J, Wang Z, Chen H (2021) Stevens-Johnson syndrome caused by enzalutamide: a case report and literature review. Front Oncol 11:736975. https://doi.org/10.3389/fonc.2021.736975
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chakraborty, A., Khanna, A., Vasudeo, V. et al. Enzalutamide-Induced Acute Maculopapular Rash in Treatment of Metastatic Prostate Cancer: First Case Report from a Tertiary Cancer Care Center of North India. Indian J Surg Oncol 14, 571–575 (2023). https://doi.org/10.1007/s13193-023-01719-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-023-01719-7