Abstract
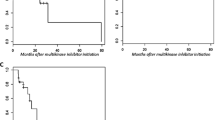
The real-world patterns of TKI use in differentiated thyroid cancer (DTC) are largely governed by the accessibility and financial feasibility of the patient with more sorafenib use compared to lenvatinib. There are limited data available on the toxicity profile, safety and tolerance of sorafenib and lenvatinib in DTC. Hence, we audited our practice on DTC. This is a retrospective single-centre analysis of patients with DTC who were referred to the Department of Medical Oncology for systemic therapy. Baseline demographics (age, sex, ECOG PS, comorbidities, substance use), tumour details (site of metastasis), previous treatment details, clinical features at metastasis (symptoms), the pattern of treatment, adverse events and outcomes including progression and death were extracted. There were 67 patients with DTC referred for systemic therapy; the median age was 56 (33–81) with a male preponderance (55.6%). The most common reason to start TKI therapy was radioactive iodine (RAI) cumulative dose > 600 milliCurie, followed by low iodine uptake in the RAI low-dose scan done at progression. The most common TKI used in the first line was sorafenib in 56 (83.6%) patients followed by lenvatinib in 9 (13.4%) patients. Papillary thyroid carcinoma was the most common histology (51, 76.1%), and the rest were follicular carcinoma (16, 23.9%). With a median follow-up of 36 months, the median PFS was 13.2 months (95% CI 10.4–16.0). The median OS was 18.8 months (95% CI 10.0–27.6). Among variables tested, no factors had a significant impact on the PFS or OS. The most common adverse events were hand-foot syndrome (54, 80.5%), diarrhoea (23, 33.3%) and transaminitis (24, 34.4%). The pattern of care of patients with RAI-refractory DTC is TKI therapy, especially sorafenib and lenvatinib in the real-world settings with comparable efficacy and safety profile compared to international literature.



Similar content being viewed by others
References
Kitahara CM, Sosa JA (2016) The changing incidence of thyroid cancer. Nat Rev Endocrinol 12(11):646–653
Gillanders SL, O’Neill JP (2018) Prognostic markers in well differentiated papillary and follicular thyroid cancer (WDTC). Eur J Surg Oncol 44(3):286–296
Schmidbauer B, Menhart K, Hellwig D, Grosse J. Differentiated thyroid cancer-treatment: state of the art. Int J Mol Sci. 2017;18(6). Available from: https://doi.org/10.3390/ijms18061292
Pacini F, Ito Y, Luster M, Pitoia F, Robinson B, Wirth L (2012) Radioactive iodine-refractory differentiated thyroid cancer: unmet needs and future directions. Expert Rev Endocrinol Metab 7(5):541–554
Thomas L, Lai SY, Dong W, Feng L, Dadu R, Regone RM et al (2014) Sorafenib in metastatic thyroid cancer: a systematic review. Oncologist 19(3):251–258
Ferrari SM, Ruffilli I, Centanni M, Virili C, Materazzi G, Alexopoulou M et al (2018) Lenvatinib in the therapy of aggressive thyroid cancer: state of the art and new perspectives with patents recently applied. Recent Pat Anticancer Drug Discov 13(2):201–208
Jayarangaiah A, Sidhu G, Brown J, Barrett-Campbell O, Bahtiyar G, Youssef I et al (2019) Therapeutic options for advanced thyroid cancer. Int J Clin Endocrinol Metab 5(1):26–34
Hirsch D, Levy S, Tsvetov G, Gorshtein A, Slutzky-Shraga I, Akirov A et al (2017) Long-term outcomes and prognostic factors in patients with differentiated thyroid cancer and distant metastases. Endocr Pract 23(10):1193–1200
Raue F, Frank-Raue K (2016) Thyroid cancer: risk-stratified management and individualized therapy. Clin Cancer Res 22(20):5012–5021
Viola D, Valerio L, Molinaro E, Agate L, Bottici V, Biagini A et al (2016) Treatment of advanced thyroid cancer with targeted therapies: ten years of experience. Endocr Relat Cancer 23(4):R185-205
Gallo M, Michelon F, Castiglione A, Felicetti F, Viansone AA, Nervo A et al (2015) Sorafenib treatment of radioiodine-refractory advanced thyroid cancer in daily clinical practice: a cohort study from a single center. Endocrine 49(3):726–734
Schneider TC, Abdulrahman RM, Corssmit EP, Morreau H, Smit JWA, Kapiteijn E (2012) Long-term analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase II trial. Eur J Endocrinol 167(5):643–650
Ye X, Zhu Y, Cai J (2015) Relationship between toxicities and clinical benefits of newly approved tyrosine kinase inhibitors in thyroid cancer: a meta-analysis of literature. J Cancer Res Ther 11(Suppl 2):C185–C190
Wilson L, Huang W, Chen L, Ting J, Cao V (2017) Cost effectiveness of lenvatinib, sorafenib and placebo in treatment of radioiodine-refractory differentiated thyroid cancer. Thyroid 27(8):1043–1052
Worden F, Fassnacht M, Shi Y, Hadjieva T, Bonichon F, Gao M et al (2015) Safety and tolerability of sorafenib in patients with radioiodine-refractory thyroid cancer. Endocr Relat Cancer 22(6):877–887
Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R et al (2015) Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372(7):621–630
Fleeman N, Houten R, Bagust A, Richardson M, Beale S, Boland A et al (2020) Lenvatinib and sorafenib for differentiated thyroid cancer after radioactive iodine: a systematic review and economic evaluation. Health Technol Assess 24(2):1–180
Fleeman N, Houten R, Chaplin M, Beale S, Boland A, Dundar Y et al (2019) A systematic review of lenvatinib and sorafenib for treating progressive, locally advanced or metastatic, differentiated thyroid cancer after treatment with radioactive iodine. BMC Cancer 19(1):1209
Feng G, Luo Y, Zhang Q, Zeng F, Xu J, Zhu J (2020) Sorafenib and radioiodine-refractory differentiated thyroid cancer (RR-DTC): a systematic review and meta-analysis. Endocrine 68(1):56–63
Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L et al (2014) Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384(9940):319–328
Kim M, Kim TH, Shin DY, Lim DJ, Kim EY, Kim WB et al (2018) Tertiary care experience of sorafenib in the treatment of progressive radioiodine-refractory differentiated thyroid carcinoma: a Korean multicenter study. Thyroid 28(3):340–348
Oh H-S, Shin DY, Kim M, Park SY, Kim TH, Kim BH et al (2019) Extended real-world observation of patients treated with sorafenib for radioactive iodine-refractory differentiated thyroid carcinoma and impact of lenvatinib salvage treatment: a korean multicenter study. Thyroid 29(12):1804–1810
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Amit Kumar Choudhary and George Abraham are co-first authors
Rights and permissions
About this article
Cite this article
Choudhary, A.K., Abraham, G., Patil, V.M. et al. Audit of Demographics, Treatment Patterns and Outcomes of Differentiated Thyroid Cancers Treated with Tyrosine Kinase Inhibitors. Indian J Surg Oncol 13, 81–86 (2022). https://doi.org/10.1007/s13193-021-01445-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-021-01445-y