Abstract
Cyanide causes severe cardiac toxicity resulting in tachycardia, hypotension, and cardiac arrest; however, the clinical diagnosis can be difficult to make. A clinical finding that may precede or predict cyanide-induced hypotension may be a trigger to provide treatment earlier and improve outcomes in cyanide toxicity. Our primary objective was to determine if there is a clinically significant change in ST segment deviation measured on ECG during intravenous cyanide infusion that may predict cyanide-induced hypotension. As part of a larger study comparing antidotes for cyanide-induced shock, 30 swine were anesthetized and monitored and then intoxicated with a continuous cyanide infusion until severe hypotension (50 % of baseline mean arterial pressure) occurred. ECGs were obtained at baseline, every 5 min during infusion, and at the development of hypotension. Repeated measures of analysis of variance were used to determine significance. The mean weight for the 30 swine at baseline was 48 kg (range 45–52), pulse rate 86 beats/min (range 55–121), and systolic blood pressure 109 mmHg (range 90–121). The mean time to hypotension was 31 min (range 16–39). The mean amount of cyanide infused was 5 mg/kg (range 2.5–6.3 mg/kg). All animals (30/30) had ECG changes in repolarization or depolarization during cyanide infusion. Significant rhythm, repolarization, and conduction changes from baseline were observed prior to severe hypotension (p < 0.05). Normal sinus rhythm and sinus tachycardia were the most common rhythms preceding hypotension. We observed ST segment elevation in leads V3, V4, III, and aVF and ST segment depression in leads aVL and aVR. The most pronounced ST segment elevation was observed in leads V3 and V4. We also detected significant changes with increased pulse rate, prolonged PR interval, and shortened QTc interval. Other significant changes were increased T axis and reduced QRS axis. We detected ST segment deviations occurring just before the onset of cyanide-induced hypotension in our swine model. Leads V3 and V4 had the most pronounced with ST elevation, but we also detected electrocardiographic ST elevation inferiorly. Shortening of the QTc and lengthening of the PR interval were also detected before hypotension.
Similar content being viewed by others
Background
Cyanide is lethal and the most common human exposure during enclosed structural fires, where cyanide is released from burning synthetic polymers and natural materials [1]. Approximately 6,000 people die in building fires each year, and recent evidence suggests that cyanide inhalation likely contributes to many of the immediate deaths [2, 3]. Industrial exposure and intentional poisonings also occur. In addition, terrorist attempts have involved cyanide, as it is inexpensive, lethal, and easily obtained [1].
There is no clear cyanide toxidrome; therefore, clinicians rely on history and suspicion to diagnose cyanide toxicity. The diagnosis may be further obscured in critically ill patients who cannot provide a history due to altered mental status. Late findings of toxicity are a triad of coma, hypotension, and severe acidosis, followed by cardiac arrest. Additional clinical findings or studies that precede hypotension or predict it may trigger the physician to provide treatments earlier and improve outcomes in cyanide toxicity.
Cardiac dysfunction during toxicity has been reported as initial tachycardia and hypertension, followed by progressive bradycardia, atrioventricular block, ventricular dysrhythmias, hypotension, and finally cardiac arrest [1]. Fortin et al [4] described asystole and supraventricular tachycardia (SVT) as the most common cardiac disorders in a retrospective study of critically ill patients with suspected cyanide toxicity. However, the authors did not determine if the dysrhythmias occurred before hypotension or arrest, and they did not report other, specific electrocardiographic interval measurements. Since electrocardiograms (ECGs) and cardiac monitoring are commonly used during the early workup of a critically ill patient and given that cyanide toxicity is a time-critical diagnosis, the potential for an earlier recognition of cyanide toxicity may be promising if the course of cardiac dysfunction and rhythm can be better described. To our knowledge, there are no reported experiments that analyzed specific electrocardiographic changes during acute, severe cyanide toxicity.
Objectives
Our primary objective was to determine if there is a clinically significant change in ST segment elevation or depression measured on ECG during intravenous cyanide infusion that may predict cyanide-induced severe hypotension. Our secondary objective was to determine if there are clinically significant changes in other ECG measurements, including pulse rate, axis, and PR and QRS intervals.
Methods
We conducted a prospective study in a swine model of cyanide toxicity as part of a larger study, comparing antidotes for cyanide-induced shock. Detailed methods of the induction of cyanide toxicity in the model have been previously described [7]. Our Institutional Animal Care and Use Committee approved the investigation. Care and handling of animals were in accord with the Office of Laboratory Animal Welfare for ethical animal research. The facilities are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Thirty Yorkshire swine (Sus scrofa) of both sexes were premedicated with ketamine (10 mg/kg), and then general anesthesia was induced and maintained with isoflurane. The animals were mechanically ventilated with a volume-limited, time-cycled ventilator (Fabius GS anesthesia machine; Drager-Siemens, New York, NY). Lead II of the surface ECG was monitored continuously.
Invasive hemodynamic parameters and core temperature were measured with a pulmonary artery catheter and monitor [5]. Aortic pressure was measured continuously through the femoral artery.
Animals were acclimated for 10 min before the experiment. A potassium cyanide mixture (3 %, normal saline) was infused at 0.16 mg/kg/min until severe hypotension occurred, which we defined as a 50 % reduction in baseline mean arterial pressure for 1 min. In our previous experiments, we found this dose effective in causing severe hypotension and 100 % lethal if untreated, but allowing animals to recover with antidotal treatment [5].
We obtained ECGs at baseline and every 5 min during cyanide infusion using a ZOLL M Series cardiac monitor and defibrillator (Zoll Medical Corporation, Chelmsford, MA) with 12-lead ECG acquisition and analysis capability. The monitor's embedded analysis program (GE Medical Systems Information Technologies 12SL™) provided a measurements matrix for every ECG, which displayed measurements of each electrocardiographic segment (e.g., QRS, QTC, PR, etc.) of each lead. Each ECG was also reviewed and interpreted by a physician. Poor quality ECGs, usually due to lead displacement or significant artifact, were excluded from the analysis. A discrepancy between the computerized reading and one investigator (T.M.) was reconciled by a second investigator (V.B.). Also as part of the larger study, we measured additional lab values including serum potassium concentrations during cyanide infusion.
A priori, we divided ECG abnormalities into three categories: rhythm, repolarization, and conduction [4]. Rhythm disorders included sinus tachycardia, dysrhythmias, and supraventricular tachycardia. Repolarization disorders included T wave abnormalities (biphasic, flattened, or inverted T waves) and ST elevation or depression. Conduction disorders included bundle branch blocks and prolonged QT intervals.
At severe hypotension, animals were randomized to receive either hydroxocobalamin or hydroxylamine as an antidote. Because our study focus was ECG changes during the period of toxicity leading to hypotension and not a comparison of antidotes, we did not evaluate ECG changes after hypotension or during antidote administration. We also did not compare outcomes of animals that survived versus those that lived through the experiment. All animals were euthanized at the end of the experiment.
We considered a 30 % change from baseline clinically significant for ST interval changes and a 20 % change for all other parameters. Differences among means were tested by a mixed effects repeated measures analysis of variance. For significant tests, two-tailed t tests were used to investigate differences among measurement times. The Holm's method was used to correct the level of significance for multiple comparisons [6]. Proportions of disorders among time intervals were evaluated using contingency tables employing the chi-square or Fisher's exact test if expected frequencies were less than five. Best estimates and 95 % confidence intervals of the proportions were calculated using Laplace's method and adjusted Wald interval, respectively. The data were evaluated for normal distribution.
Results
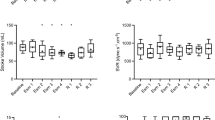
At baseline, the mean weight of the swine was 48 kg (range 45–52), pulse rate was 86 beats/min (range 55–121), and systolic blood pressure was 109 mmHg (range 90–121). The baseline serum potassium was 3.45 mEq/L (range 3–3.8). Baseline ECG abnormalities were detected (Table 1). All 30 animals reached severe hypotension within 40 min of cyanide infusion (mean 31 min, range 16–39). The mean cyanide dose infused was 5 mg/kg (2.5–6.3 mg/kg). All (30/30) animals showed ECG changes prior to hypotension. The mean potassium at hypotension was 4.59 mEq/L (range 3.4–4.6). All three categories of disorders (rhythm, repolarization, and conduction) had significant changes from baseline measurements (Table 1). All animals had normal sinus rhythm or sinus tachycardia until hypotension.
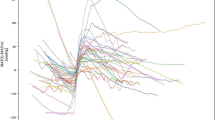
We evaluated ST deviations independently and detected significant differences from baseline in leads V3, V4, III, aVL, aVR, and aVF (Figs. 1 and 2a, b, c, d). ST elevation occurred in leads V3, V4, III, and aVF and ST depression occurred in aVR and aVL. Most ST deviations (80 %) occurred at 25 min of infusion, and the greatest deviations occurred at 30 min of infusion. This correlates to a mean cyanide dose of 3.2 mg/kg at 20 min, 4.0 mg/kg at 25 min, and 4.8 mg/kg at 30 min.
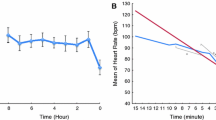
Graphs of electrocardiographic findings found to be significantly different between baseline and hypotension. Cyanide infusion was initiated at baseline. HypoTN hypotension, min minute, mm millimeter. a ST segment elevation over time. b Pulse rate over time. c PR segment interval length over time. d QTc segment interval length over time
In addition, we observed that pulse rate (p = 0.0003), QRS axis (p < 0.0001), T wave axis (p = 0.0125), PR interval (p < 0.0001), and QTc interval (p = 0.0004) were significantly different from baseline, using RMANOVA to analyze the differences (Table 2). Because 31 min was the mean time to hypotension, we reported the differences in intervals from baseline compared to 30 min of cyanide infusion. Heart rate increased immediately and was 14 % greater than baseline at 30 min. PR interval prolongation began at 5 min and continued through 30 min and until hypotension. Despite increased heat rate at 5 min, QTc interval shortened throughout the course until hypotension. QRS interval was not significantly different. ST depression in V2 and V3 (Fig. 1) occurred, but was not statistically significant.
Discussion
In our study, we observed that clinically significant ECG changes occurred during acute cyanide toxicity. We detected not only ST segment changes, but also changes in heart rate, QRS axis, T axis, PR interval, and QTc interval. Notably, all of these changes occurred prior to the onset of severe hypotension. Detecting significant ECG changes early in the clinical course and prior to hypotension may be a predictor of cyanide-induced hypotension and an early marker for pending cardiac arrest. Combined with other clinical signs, such as hypotension, coma, and lactic acidosis, recognizable ECG changes may predict cardiovascular collapse from cyanide.
The more pronounced ST deviations occurred in the anterior leads of V3 and V4, but ST elevation was also detected in leads aVF and III, with ST depression in leads aVL and aVR. This group of leads implies a global cardiac effect. The ST changes increased the most at 30 min of cyanide, after approximately 4.8 mg/kg and approximately 5 min before hypotension.
Cyanide inhibits multiple enzyme systems, most importantly, cytochrome oxidase at cytochrome a3, which is the last step in the mitochondrial electron transport chain. This uncouples oxidative phosphorylation and disrupts the efficient aerobic production of ATP. Anaerobic ATP production then dominates through conversion of glucose to pyruvate and then to lactate. Systemic acidosis is associated with a large rise in serum lactate, and more importantly, the acidosis is due to the hydrogen ion production from cellular metabolism without the balancing process of oxidative phosphorylation [1].
Organ systems that use large amounts of ATP, especially, cardiac and central nervous system tissues, are most affected by the inhibition of aerobic metabolism. Postmortem studies have shown highest cyanide concentrations in the brain and heart [1]. ATP deficits reduce Na+/K + ATPase pump activity, shifting the Na+/K + gradient as less potassium is pumped back into cells during repolarization. This impairment of sodium and potassium could explain the ST segment changes. This may explain the common peaked T waves (increased T axis) and rise in serum potassium we observed during the experiment. QTc segments did shorten over the course of cyanide infusion. This effect could partially be explained by increased heart rate; however, the heart rate increased immediately, and the QTc length shortened over time. In addition, the potassium increased over time and may reduce the QTc interval; however, potassium levels are not always a direct correlation to QTc length [7]. Reduced ion pump activity can prolong repolarization and impair conduction, producing cardiac dysfunction similar to diffuse ischemia from other causes. This mechanism may account for the ECG changes we observed during the experiment [4]. As in our study, Aberra [8] reported PR prolongation in an in vitro isolated rat heart model with acidosis, resulting in similar finding as our study. However, the model was exposed to a pH of 6.5 which is much lower than the lowest serum pH, in our study, which was of 7.2. In addition, in Aberra's acidotic in vitro rat model, changes in QT interval, ST change, and QRS interval did not significantly change. Moréna [9] evaluated an in vitro porcine heart model with severe acidosis (pH 6.8), hyperkalemia (up to 10 meq/L), hypoglycemia, and hypoxia and reported that ECG electrical changes were not directly related to metabolic changes alone, which is congruent with our assumption that cyanide induced most of the ECG changes we detected.
Our findings are similar to a previous retrospective study of human cyanide toxicity cases by Fortin et al [4]. who also documented cardiac dysfunction including rhythm, repolarization, and conduction disorders. However, SVT was more prevalent in their retrospective review, whereas we more commonly detected sinus tachycardia. In addition, Fortin did not report rhythms detected before hypotension or cardiac arrest, and the authors did not report other ECG intervals. We reported correlation between observed ECG effects and time of exposure, cyanide dose, and cardiovascular effects.
Limitations
There are several limitations to this animal study. An animal model does not exactly replicate human toxicity or response. However, the swine model has been used successfully in previous investigations of cyanide toxicity and most closely models human cardiovascular anatomy and physiology [10, 11]. Also, the standard computer measurements provided in the measurements matrix were inaccurate on some ECGs. We reviewed every ECG manually and reconciled discrepancies between researcher and computer interpretations. Another limitation was the use of intravenous cyanide rather than the more common inhalational exposure. Both routes provide rapid toxicity, but intravenous delivery ensured a more controlled exposure to the animals, while minimizing the risk to research staff from undetected leaks in the ventilation system [12, 13]. We minimized this limitation by establishing a clinical end point of severe hypotension and ensuring that toxic levels similar to those observed in humans were achieved [5]. We have used potassium cyanide for this and our previous study; however, the potassium infused is small, approximately 1 mEq of potassium infused over 30 min. Finally, we did not correlate serum cyanide levels with specific ECG abnormalities, although this would not change current clinical practice without a common and rapid method of measuring cyanide levels. Nevertheless, we did correlate ECG findings with cyanide dose infused.
Conclusion
In our swine study, we detected ST segment deviations occurring just before the onset of cyanide-induced hypotension. Leads V3 and V4 were most affected by ST elevation, but we also detected electrocardiographic ST elevation inferiorly. Shortening of the QTc and lengthening of the PR interval were also detected before hypotension.
References
Morocco AP (2005) Cyanides. Crit Care Clin 21(4):691–705, vi
Istre GR, McCoy MA, Osborn L, Barnard JJ, Bolton A (2001) Deaths and injuries from house fires. N Engl J Med 344(25):1911–1916
Borron SW, Baud FJ, Barriot P, Imbert M, Bismuth C (2007) Prospective study of hydroxocobalamin for acute cyanide poisoning in smoke inhalation. Ann Emerg Med 49(6):794–801, 801.e791–792
Fortin JL, Desmettre T, Manzon C et al (2010) Cyanide poisoning and cardiac disorders: 161 cases. J Emerg Med 38(4):467–476
Bebarta VS, Tanen DA, Lairet J, Dixon PS, Valtier S, Bush A (2010) Hydroxocobalamin and sodium thiosulfate versus sodium nitrite and sodium thiosulfate in the treatment of acute cyanide toxicity in a swine (Sus scrofa) model. Ann Emerg Med 55(4):345–351
Holm S (1979) A simple sequential rejective multiple test procedure. Scand J Statistics 6:65–70
Nelson LS (2002) Toxicologic myocardial sensitization. J Toxicol Clin Toxicol 40(7):867–879
Aberra A, Komukai K, Howarth FC, Orchard CH (2001) The effect of acidosis on the ECG of the rat heart. Exp Physiol 86(1):27–31
Moréna H, Janse MJ, Fiolet JW, Krieger WJ, Crijns H, Durrer D (1980) Comparison of the effects of regional ischemia, hypoxia, hyperkalemia, and acidosis on intracellular and extracellular potentials and metabolism in the isolated porcine heart. Circ Res 46(5):634–646
Idris AH, Becker LB, Ornato JP et al (1996) Utstein-style guidelines for uniform reporting of laboratory CPR research. A statement for healthcare professionals from a task force of the American Heart Association, the American College of Emergency Physicians, the American College of Cardiology, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, the Institute of Critical Care Medicine, the Safar Center for Resuscitation Research, and the Society for Academic Emergency Medicine. Writing group. Circulation 94(9):2324–2336
Hannon JP, Bossone CA, Wade CE (1990) Normal physiological values for conscious pigs used in biomedical research. Lab Anim Sci 40(3):293–298
Vick J, Marino MT, von Bredow JD, Kaminskis A, Brewer T (2000) A reproducible nonlethal animal model for studying cyanide poisoning. Mil Med 165(12):967–972
Borron SW, Stonerook M, Reid F (2006) Efficacy of hydroxocobalamin for the treatment of acute cyanide poisoning in adult beagle dogs. Clin Toxicol (Phila) 44(Suppl 1):5–15
Acknowledgments
The US Air Force Office of the Surgeon General funded this study. No other funding was used. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the US Air Force, Department of Defense, or the US government.
Conflict of Interest
There is no prior publication, conflict of interest, or copyright constraint.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was presented at the US Air Force Medical Services meeting, 2010.
Rights and permissions
About this article
Cite this article
Muncy, T.A., Bebarta, V.S., Varney, S.M. et al. Acute Electrocardiographic ST Segment Elevation May Predict Hypotension in a Swine Model of Severe Cyanide Toxicity. J. Med. Toxicol. 8, 285–290 (2012). https://doi.org/10.1007/s13181-012-0226-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13181-012-0226-2