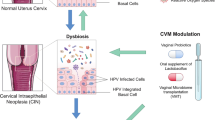
Abstract
HPVs representing the most common sexually transmitted disease are a group of carcinogenic viruses with different oncogenic potential. The immune system and the vaginal microbiome represent the modifiable and important risk factors in HPV-induced carcinogenesis. HPV infection significantly increases vaginal microbiome diversity, leading to gradual increases in the abundance of anaerobic bacteria and consequently the severity of cervical dysplasia. Delineation of the exact composition of the vaginal microbiome and immune environment before HPV acquisition, during persistent/progressive infections and after clearance, provides insights into the complex mechanisms of cervical carcinogenesis. It gives hints regarding the prediction of malignant potential. Relative high HPV prevalence in the general population is a challenge for modern and personalized diagnostics and therapeutic guidelines. Identifying the dominant microbial biomarkers of high-grade and low-grade dysplasia could help us to triage the patients with marked chances of lesion regression or progression. Any unnecessary surgical treatment of cervical dysplasia could negatively affect obstetrical outcomes and sexual life. Therefore, understanding the effect and role of microbiome-based therapies is a breaking point in the conservative management of HPV-associated precanceroses. The detailed evaluation of HPV capabilities to evade immune mechanisms from various biofluids (vaginal swabs, cervicovaginal lavage/secretions, or blood) could promote the identification of new immunological targets for novel individualized diagnostics and therapy. Qualitative and quantitative assessment of local immune and microbial environment and associated risk factors constitutes the critical background for preventive, predictive, and personalized medicine that is essential for improving state-of-the-art medical care in patients with cervical precanceroses and cervical cancer. The review article focuses on the influence and potential diagnostic and therapeutic applications of the local innate immune system and the microbial markers in HPV-related cancers in the context of 3P medicine.


Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Golubnitschaja O, Costigliola V, EPMA. General report & recommendations in predictive, preventive and personalised medicine. white paper of the European Association for Predictive. Prev Personal Med EPMA J. 2012;2012(3):14. https://doi.org/10.1186/1878-5085-3-14.
Golubnitschaja O, Yeghiazaryan K, Costigliola V, Trog D, Braun M, Debald M, et al. Risk assessment, disease prevention and personalised treatments in breast cancer: is clinically qualified integrative approach in the horizon? EPMA J. 2013;4:6. https://doi.org/10.1186/1878-5085-4-6.
Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health Elsevier. 2020;8:e191-203. https://doi.org/10.1016/S2214-109X(19)30482-6.
Stelzle D, Tanaka LF, Lee KK, Khalil AI, Baussano I, Shah ASV, et al. Estimates of the global burden of cervical cancer associated with HIV. The Lancet Global Health Elsevier. 2021;9:e161–9. https://doi.org/10.1016/S2214-109X(20)30459-9.
Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393:169–82. https://doi.org/10.1016/S0140-6736(18)32470-X.
zur Hausen H. Papillomaviruses in the causation of human cancers — a brief historical account. Virology. 2009;384:260–5. https://doi.org/10.1016/j.virol.2008.11.046.
Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, Ghissassi FE, et al. A review of human carcinogens—Part B: biological agents. Lancet Oncol. 2009;10:321–2. https://doi.org/10.1016/S1470-2045(09)70096-8.
Halec G, Alemany L, Lloveras B, Schmitt M, Alejo M, Bosch FX, et al. Pathogenic role of the eight probably/possibly carcinogenic HPV types 26, 53, 66, 67, 68, 70, 73 and 82 in cervical cancer. J Pathol. 2014;234:441–51. https://doi.org/10.1002/path.4405.
Bosch FX, de Sanjosé S. Chapter 1: Human papillomavirus and cervical cancer--burden and assessment of causality. J Natl Cancer Inst Monogr. 2003;3–13. https://doi.org/10.1093/oxfordjournals.jncimonographs.a003479.
Jaisamrarn U, Castellsagué X, Garland SM, Naud P, Palmroth J, Del Rosario-Raymundo MR, et al. Natural history of progression of HPV infection to cervical lesion or clearance: analysis of the control arm of the large, randomised PATRICIA study. PLoS ONE. 2013;8:e79260. https://doi.org/10.1371/journal.pone.0079260.
Burchell AN, Winer RL, de Sanjosé S, Franco EL. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24(Suppl 3):S3/52-61. https://doi.org/10.1016/j.vaccine.2006.05.031.
Demarco M, Lorey TS, Fetterman B, Cheung LC, Guido RS, Wentzensen N, et al. Risks of CIN 2+, CIN 3+, and cancer by cytology and human papillomavirus status: the foundation of risk-based cervical screening guidelines. J Low Genit Tract Dis. 2017;21:261–7. https://doi.org/10.1097/LGT.0000000000000343.
Demarco M, Egemen D, Raine-Bennett TR, Cheung LC, Befano B, Poitras NE, et al. A study of partial human papillomavirus genotyping in support of the 2019 ASCCP risk-based management consensus guidelines. J Low Genit Tract Dis. 2020;24:144–7. https://doi.org/10.1097/LGT.0000000000000530.
Sasieni P, Castanon A, Landy R, Kyrgiou M, Kitchener H, Quigley M, et al. Risk of preterm birth following surgical treatment for cervical disease: executive summary of a recent symposium. BJOG. 2016;123:1426–9. https://doi.org/10.1111/1471-0528.13839.
Liskova Alena, Samec Marek, Koklesova Lenka, Kudela Erik, Kubatka Peter, Golubnitschaja Olga. Mitochondriopathies as a clue to systemic disorders: “vicious circle” of mitochondrial injury, analytical tools and mitigating measures in context of predictive, preventive, and personalized (3P) medicine. 2021; 18;22(4):2007 https://doi.org/10.3390/ijms22042007.
Crigna AT, Samec M, Koklesova L, Liskova A, Giordano FA, Kubatka P, et al. Cell-free nucleic acid patterns in disease prediction and monitoring-hype or hope? EPMA J. 2020;1–25. https://doi.org/10.1007/s13167-020-00226-x.
Liskova A, Samec M, Koklesova L, Giordano FA, Kubatka P, Golubnitschaja O. Liquid biopsy is instrumental for 3PM dimensional solutions in cancer management. J Clin Med Multidiscip Digital Publishing Institute. 2020;9:2749. https://doi.org/10.3390/jcm9092749.
Gerner C, Costigliola V, Golubnitschaja O. Multiomic patterns in body fluids: technological challenge with a great potential to implement the advances paradigm of 3P medicine. Mass Spectrom Rev. 2019. https://doi.org/10.1002/mas.21612.
Zoodsma M, Sijmons RH, de Vries EG, van der Zee AG. Familial cervical cancer: case reports, review and clinical implications. Hereditary Cancer Clin Pract. 2004;2:99. https://doi.org/10.1186/1897-4287-2-2-99.
Oyervides-Muñoz MA, Pérez-Maya AA, Sánchez-Domínguez CN, Berlanga-Garza A, Antonio-Macedo M, Valdéz-Chapa LD, et al. Multiple HPV infections and viral load association in persistent cervical lesions in Mexican women. Viruses [Internet]. 2020 [cited 2021 Feb 21];12. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7232502/. https://doi.org/10.3390/v12040380.
Franceschi S, Herrero R, Clifford GM, Snijders PJF, Arslan A, Anh PTH, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer. 2006;119:2677–84. https://doi.org/10.1002/ijc.22241.
Wendland EM, Villa LL, Unger ER, Domingues CM, Benzaken AS. Prevalence of HPV infection among sexually active adolescents and young adults in Brazil: the POP-Brazil study. Scientific Reports. Nature Publishing Group. 2020;10:4920. https://doi.org/10.1038/s41598-020-61582-2.
Río-Ospina LD, León SCSD, Camargo M, Sánchez R, Mancilla CL, Patarroyo ME, et al. The prevalence of high-risk HPV types and factors determining infection in female Colombian adolescents. PLOS ONE Public Library of Science. 2016;11:e0166502. https://doi.org/10.1371/journal.pone.0166502.
Giroux V, Rustgi AK. Metaplasia: tissue injury adaptation and a precursor to the dysplasia–cancer sequence. Nat Rev Cancer. 2017;17:594–604. https://doi.org/10.1038/nrc.2017.68.
Bedin R, Gasparin VA, Pitilin ÉDB. Fatores associados às alterações cérvico-uterinas de mulheres atendidas em um município polo do oeste catarinense Factors associated to uterine-cervix changes in women assisted in a pole town in western Santa Catarina. R pesq cuid fundam online. 2017;9:167. https://doi.org/10.9789/2175-5361.2017.v9i1.167-174.
Kang L-N, Castle PE, Zhao F-H, Jeronimo J, Chen F, Bansil P, et al. A prospective study of age trends of high-risk human papillomavirus infection in rural China. BMC Infect Dis. 2014;14:96. https://doi.org/10.1186/1471-2334-14-96.
Muñoz N, Franceschi S, Bosetti C, Moreno V, Herrero R, Smith JS, et al. Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case-control study. Lancet. 2002;359:1093–101. https://doi.org/10.1016/S0140-6736(02)08151-5.
Moreno V, Bosch FX, Muñoz N, Meijer CJLM, Shah KV, Walboomers JMM, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359:1085–92. https://doi.org/10.1016/S0140-6736(02)08150-3.
Orlando G, Tanzi A, Rizzardini G. Modifiable and non-modifiable factors related to HPV infection and cervical abnormalities in women at high risk: a cross-sectional analysis from the VALHIDATE study. 2016;21. Available from: https://air.unimi.it/handle/2434/424485#.YI1A3bUzaUk. Accessed 01 July 2016.
Roura E, Travier N, Waterboer T, de Sanjosé S, Bosch FX, Pawlita M, et al. The influence of hormonal factors on the risk of developing cervical cancer and pre-cancer: results from the EPIC cohort. PLoS One [Internet]. 2016 [cited 2021 Mar 3];11. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4726518/. https://doi.org/10.1371/journal.pone.0147029.
Asthana S, Busa V, Labani S. Oral contraceptives use and risk of cervical cancer—a systematic review & meta-analysis. Eur J Obstet & Gynecol Reprod Biol. 2020;247:163–75. https://doi.org/10.1016/j.ejogrb.2020.02.014.
Mignot S, Ringa V, Vigoureux S, Zins M, Panjo H, Saulnier P-J, et al. Pap tests for cervical cancer screening test and contraception: analysis of data from the CONSTANCES cohort study. BMC Cancer. 2019;19:317. https://doi.org/10.1186/s12885-019-5477-8.
Torres-Poveda K, Ruiz-Fraga I, Madrid-Marina V, Chavez M, Richardson V. High risk HPV infection prevalence and associated cofactors: a population-based study in female ISSSTE beneficiaries attending the HPV screening and early detection of cervical cancer program. BMC Cancer. 2019;19:1205. https://doi.org/10.1186/s12885-019-6388-4.
Fonseca-Moutinho JA. Smoking and cervical cancer. ISRN Obstet Gynecol [Internet]. 2011 [cited 2021 Mar 3];2011. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3140050/. https://doi.org/10.5402/2011/847684.
Oh HY, Kim MK, Seo S, Lee DO, Chung YK, Lim MC, et al. Alcohol consumption and persistent infection of high-risk human papillomavirus. Epidemiol Infect. 2015;143:1442–50. https://doi.org/10.1017/S0950268814002258.
Minkoff H, Zhong Y, Strickler HD, Watts DH, Palefsky JM, Levine AM, et al. The relationship between cocaine use and human papillomavirus infections in HIV-seropositive and HIV-seronegative women. Infect Dis Obstet Gynecol. 2008;2008:587082 https://doi.org/10.1155/2008/587082
Shah SC, Kayamba V, Peek RM, Heimburger D. Cancer control in low- and middle-income countries: is it time to consider screening? JGO. Wolters Kluwer; 2019;1–8. https://doi.org/10.1200/JGO.18.00200.
Liu Z, Ren Z, Zhang J, Chuang C-C, Kandaswamy E, Zhou T, et al. Role of ROS and nutritional antioxidants in human diseases. Front Physiol [Internet]. 2018 [cited 2021 Mar 3];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5966868/. https://doi.org/10.3389/fphys.2018.00477.
Koshiyama M. The effects of the dietary and nutrient intake on gynecologic cancers. Healthcare (Basel) [Internet]. 2019 [cited 2021 Mar 3];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6787610/. https://doi.org/10.3390/healthcare7030088.
Castanheira CP, Sallas ML, Nunes RAL, Lorenzi NPC, Termini L. Microbiome and cervical cancer. Pathobiology. 2020;1–11. https://doi.org/10.1159/000511477.
Lavitola G, Della Corte L, De Rosa N, Nappi C, Bifulco G. Effects on vaginal microbiota restoration and cervical epithelialization in positive HPV patients undergoing vaginal treatment with carboxy-methyl-beta-glucan. Biomed Res Int. 2020;2020:1–8. https://doi.org/10.1155/2020/5476389.
Godha K, Tucker KM, Biehl C, Archer DF, Mirkin S. Human vaginal pH and microbiota: an update. Gynecol Endocrinol. 2018;34:451–5. https://doi.org/10.1080/09513590.2017.1407753.
Goncharenko V, Bubnov R, Polivka J, Zubor P, Biringer K, Bielik T, et al. Vaginal dryness: individualised patient profiles, risks and mitigating measures. EPMA J. 2019;10:73–9. https://doi.org/10.1007/s13167-019-00164-3.
Muhleisen AL, Herbst-Kralovetz MM. Menopause and the vaginal microbiome. Maturitas. 2016;91:42–50. https://doi.org/10.1016/j.maturitas.2016.05.015.
Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2:4. https://doi.org/10.1186/2049-2618-2-4.
van de Wijgert JHHM, Borgdorff H, Verhelst R, Crucitti T, Francis S, Verstraelen H, et al. The vaginal microbiota: what have we learned after a decade of molecular characterization? Fredricks DN, editor. PLoS ONE. 2014;9:e105998. https://doi.org/10.1371/journal.pone.0105998.
Gajer P, Brotman RM, Bai G, Sakamoto J, Schutte UME, Zhong X, et al. Temporal dynamics of the human vaginal microbiota. Science Translational Medicine. 2012;4:132ra52-132ra52. https://doi.org/10.1126/scitranslmed.3003605.
Łaniewski P, Ilhan ZE, Herbst-Kralovetz MM. The microbiome and gynaecological cancer development, prevention and therapy. Nat Rev Urol. 2020;17:232–50. https://doi.org/10.1038/s41585-020-0286-z.
Flores R, Shi J, Fuhrman B, Xu X, Veenstra TD, Gail MH, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;10:253. https://doi.org/10.1186/1479-5876-10-253.
Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe. 2011;10:324–35. https://doi.org/10.1016/j.chom.2011.10.003.
Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci. 2011;108:4680–7. https://doi.org/10.1073/pnas.1002611107.
Boris S, Suárez JE, Vázquez F, Barbés C. Adherence of human vaginal lctobacilli to vaginal epithelial cells and interaction with uropathogens. Infect Immun. 1998;66:1985–9. https://doi.org/10.1128/IAI.66.5.1985-1989.1998.
Medina-Colorado AA, Vincent KL, Miller AL, Maxwell CA, Dawson LN, Olive T, et al. Vaginal ecosystem modeling of growth patterns of anaerobic bacteria in microaerophilic conditions. Anaerobe. 2017;45:10–8. https://doi.org/10.1016/j.anaerobe.2017.04.014.
V. Sgibnev A, A. Kremleva E. Vaginal protection by H2O2-producing lactobacilli. Jundishapur J Microbiol [Internet]. 2015 [cited 2021 Mar 7];8. Available from: https://sites.kowsarpub.com/jjm/articles/56511.html. https://doi.org/10.5812/jjm.22913.
Zadravec P, Štrukelj B, Berlec A. Improvement of LysM-mediated surface display of designed ankyrin repeat proteins (DARPins) in recombinant and nonrecombinant strains of Lactococcus lactis and Lactobacillus species. Björkroth J, editor. Appl Environ Microbiol. 2015;81:2098–106. https://doi.org/10.1128/AEM.03694-14.
Homburg C, Bommer M, Wuttge S, Hobe C, Beck S, Dobbek H, et al. Inducer exclusion in Firmicutes: insights into the regulation of a carbohydrate ATP binding cassette transporter from Lactobacillus casei BL23 by the signal transducing protein P-Ser46-HPr. Mol Microbiol. 2017;105:25–45. https://doi.org/10.1111/mmi.13680.
Wang S, Wang Q, Yang E, Yan L, Li T, Zhuang H. Antimicrobial compounds produced by vaginal Lactobacillus crispatus are able to strongly inhibit Candida albicans growth, hyphal formation and regulate virulence-related gene expressions. Front Microbiol [Internet]. 2017 [cited 2021 Mar 7];08. Available from: http://journal.frontiersin.org/article/10.3389/fmicb.2017.00564/fullhttps://doi.org/10.3389/fmicb.2017.00564.
Yang X, Da M, Zhang W, Qi Q, Zhang C, Han S. Role of Lactobacillus in cervical cancer. CMAR. 2018;10:1219–29. https://doi.org/10.2147/CMAR.S165228.
So KA, Yang EJ, Kim NR, Hong SR, Lee J-H, Hwang C-S, et al. Changes of vaginal microbiota during cervical carcinogenesis in women with human papillomavirus infection. Consolaro MEL, editor. PLoS ONE. 2020;15:e0238705. https://doi.org/10.1371/journal.pone.0238705.
Amabebe E, Anumba DOC. The vaginal microenvironment: the physiologic role of lactobacilli. Front Med. 2018;5:181. https://doi.org/10.3389/fmed.2018.00181.
O’Hanlon DE, Moench TR, Cone RA. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. Landay A, editor. PLoS ONE. 2013;8:e80074. https://doi.org/10.1371/journal.pone.0080074.
Wang H, Ma Y, Li R, Chen X, Wan L, Zhao W. Associations of cervicovaginal lactobacilli with high-risk human papillomavirus infection, cervical intraepithelial neoplasia, and cancer: a systematic review and meta-analysis. J Infect Dis. 2019;220:1243–54. https://doi.org/10.1093/infdis/jiz325.
Brotman RM, Shardell MD, Gajer P, Fadrosh D, Chang K, Silver MI, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2014;21:450–8. https://doi.org/10.1097/GME.0b013e3182a4690b.
Kyrgiou M, Mitra A, Moscicki A-B. Does the vaginal microbiota play a role in the development of cervical cancer? Transl Res. 2017;179:168–82. https://doi.org/10.1016/j.trsl.2016.07.004.
Chao X-P, Sun T-T, Wang S, Fan Q-B, Shi H-H, Zhu L, et al. Correlation between the diversity of vaginal microbiota and the risk of high-risk human papillomavirus infection. Int J Gynecol Cancer. 2019;29:28–34. https://doi.org/10.1136/ijgc-2018-000032.
Mitra A, MacIntyre DA, Lee YS, Smith A, Marchesi JR, Lehne B, et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci Rep. 2015;5:16865. https://doi.org/10.1038/srep16865.
Schwebke JR, Muzny CA, Josey WE. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: a conceptual model. J Infect Dis. 2014;210:338–43. https://doi.org/10.1093/infdis/jiu089.
Moscicki A-B, Shi B, Huang H, Barnard E, Li H. Cervical-vaginal microbiome and associated cytokine profiles in a prospective study of HPV 16 acquisition, persistence, and clearance. Front Cell Infect Microbiol. 2020;10:569022. https://doi.org/10.3389/fcimb.2020.569022.
Piyathilake CJ, Ollberding NJ, Kumar R, Macaluso M, Alvarez RD, Morrow CD. Cervical microbiota associated with higher grade cervical intraepithelial neoplasia in women infected with high-risk human papillomaviruses. Cancer Prev Res (Phila). 2016;9:357–66. https://doi.org/10.1158/1940-6207.CAPR-15-0350.
Norenhag J, Du J, Olovsson M, Verstraelen H, Engstrand L, Brusselaers N. The vaginal microbiota, human papillomavirus and cervical dysplasia: a systematic review and network meta‐analysis. BJOG. Int J Obstet Gy. 2020;127:171–80. https://doi.org/10.1111/1471-0528.15854.
Oh HY, Kim BS, Seo SS, Kong JS, Lee JK, Park SY, et al. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin Microbiol Infect. 2015;21(674):e1-9. https://doi.org/10.1016/j.cmi.2015.02.026.
Lamont RF, Morgan DJ, Wilden SD, Taylor-Robinson D. Prevalence of bacterial vaginosis in women attending one of three general practices for routine cervical cytology. Int J STD AIDS. 2000;11:495–8. https://doi.org/10.1258/0956462001916371.
Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505–23. https://doi.org/10.1016/j.ajog.2013.05.006.
Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1–137. https://doi.org/10.15585/mmwr%20.rr6404a1.
Curty G, de Carvalho PS, Soares MA. The role of the cervicovaginal microbiome on the genesis and as a biomarker of premalignant cervical intraepithelial neoplasia and invasive cervical cancer. Int J Mol Sci [Internet]. 2019 [cited 2021 Jan 27];21. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6981542/. https://doi.org/10.3390/ijms21010222.
Shannon B, Yi TJ, Perusini S, Gajer P, Ma B, Humphrys MS, et al. Association of HPV infection and clearance with cervicovaginal immunology and the vaginal microbiota. Mucosal Immunol. 2017;10:1310–9. https://doi.org/10.1038/mi.2016.129.
Wei Z-T, Chen H-L, Wang C-F, Yang G-L, Han S-M, Zhang S-L. Depiction of vaginal microbiota in women with high-risk human papillomavirus infection. Front Public Health. 2021;8:587298. https://doi.org/10.3389/fpubh.2020.587298.
Machado A, Cerca N. Influence of biofilm formation by Gardnerella vaginalis and other anaerobes on bacterial vaginosis. J Infect Dis. 2015;212:1856–61. https://doi.org/10.1093/infdis/jiv338.
Usyk M, Zolnik CP, Castle PE, Porras C, Herrero R, Gradissimo A, et al. Cervicovaginal microbiome and natural history of HPV in a longitudinal study. Silvestri G, editor. PLoS Pathog. 2020;16:e1008376. https://doi.org/10.1371/journal.ppat.1008376.
Murphy K, Mitchell CM. The interplay of host immunity, environment and the risk of bacterial vaginosis and associated reproductive health outcomes. J Infect Dis. 2016;214(Suppl 1):S29-35. https://doi.org/10.1093/infdis/jiw140.
Meng LT, Xue Y, Yue T, Yang L, Gao L, An RF. Relationship of HPV infection and BV, VVC, TV: a clinical study based on 1 261 cases of gynecologic outpatients. Zhonghua Fu Chan Ke Za Zhi. 2016;51:730–3. https://doi.org/10.3760/cma.j.issn.0529-567X.2016.10.004.
Zhou D, Cui Y, Wu FL, Deng WH. The change of vaginal lactobacillus in patients with high-risk human papillomavirus infection. Zhonghua Yi Xue Za Zhi. 2016;96:2006–8. https://doi.org/10.3760/cma.j.issn.0376-2491.2016.25.010.
Chen Y, Qiu X, Wang W, Li D, Wu A, Hong Z, et al. Human papillomavirus infection and cervical intraepithelial neoplasia progression are associated with increased vaginal microbiome diversity in a Chinese cohort. BMC Infect Dis. 2020;20:629. https://doi.org/10.1186/s12879-020-05324-9.
Godoy-Vitorino F, Romaguera J, Zhao C, Vargas-Robles D, Ortiz-Morales G, Vázquez-Sánchez F, et al. Cervicovaginal fungi and bacteria associated with cervical intraepithelial neoplasia and high-risk human papillomavirus infections in a hispanic population. Front Microbiol. 2018;9:2533. https://doi.org/10.3389/fmicb.2018.02533.
Doerflinger SY, Throop AL, Herbst-Kralovetz MM. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. J Infect Dis. 2014;209:1989–99. https://doi.org/10.1093/infdis/jiu004.
Larsen AK, Nymo IH, Briquemont B, Sørensen KK, Godfroid J. Entrance and survival of Brucella pinnipedialis hooded seal strain in human macrophages and epithelial cells. PLoS ONE. 2013;8:e84861. https://doi.org/10.1371/journal.pone.0084861.
Zhou Y, Wang L, Pei F, Ji M, Zhang F, Sun Y, et al. Patients with LR-HPV infection have a distinct vaginal microbiota in comparison with healthy controls. Front Cell Infect Microbiol. 2019;9:294. https://doi.org/10.3389/fcimb.2019.00294.
Audirac-Chalifour A, Torres-Poveda K, Bahena-Román M, Téllez-Sosa J, Martínez-Barnetche J, Cortina-Ceballos B, et al. Cervical microbiome and cytokine profile at various stages of cervical cancer: a pilot study. PLoS ONE. 2016;11:e0153274. https://doi.org/10.1371/journal.pone.0153274.
Lee JE, Lee S, Lee H, Song Y-M, Lee K, Han MJ, et al. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS ONE. 2013;8:e63514. https://doi.org/10.1371/journal.pone.0063514.
Mitra A, MacIntyre DA, Ntritsos G, Smith A, Tsilidis KK, Marchesi JR, et al. The vaginal microbiota associates with the regression of untreated cervical intraepithelial neoplasia 2 lesions. Nat Commun. 2020;11:1999. https://doi.org/10.1038/s41467-020-15856-y.
Caselli E, D’Accolti M, Santi E, Soffritti I, Conzadori S, Mazzacane S, et al. Vaginal microbiota and cytokine microenvironment in HPV clearance/persistence in women surgically treated for cervical intraepithelial neoplasia: an observational prospective study. Front Cell Infect Microbiol. 2020;10:540900. https://doi.org/10.3389/fcimb.2020.540900.
Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS ONE. 2012;7:e37818. https://doi.org/10.1371/journal.pone.0037818.
Bubnov RV, Spivak MY, Lazarenko LM, Bomba A, Boyko NV. Probiotics and immunity: provisional role for personalized diets and disease prevention. EPMA J. 2015;6:14. https://doi.org/10.1186/s13167-015-0036-0.
Reid G, Abrahamsson T, Bailey M, Bindels LB, Bubnov R, Ganguli K, et al. How do probiotics and prebiotics function at distant sites? Benef Microbes. 2017;8:521–33. https://doi.org/10.3920/BM2016.0222.
Lev-Sagie A, Goldman-Wohl D, Cohen Y, Dori-Bachash M, Leshem A, Mor U, et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med. 2019;25:1500–4. https://doi.org/10.1038/s41591-019-0600-6.
Vujic G, Jajac Knez A, Despot Stefanovic V, Kuzmic VV. Efficacy of orally applied probiotic capsules for bacterial vaginosis and other vaginal infections: a double-blind, randomized, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 2013;168:75–9. https://doi.org/10.1016/j.ejogrb.2012.12.031.
Abdelhamid AG, El-Masry SS, El-Dougdoug NK. Probiotic Lactobacillus and Bifidobacterium strains possess safety characteristics, antiviral activities and host adherence factors revealed by genome mining. EPMA J. 2019;10:337–50. https://doi.org/10.1007/s13167-019-00184-z.
Li Y, Yu T, Yan H, Li D, Yu T, Yuan T, et al. Vaginal microbiota and HPV infection: novel mechanistic insights and therapeutic strategies. IDR. 2020;13:1213–20. https://doi.org/10.2147/IDR.S210615.
Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367:489–98. https://doi.org/10.1016/S0140-6736(06)68181-6.
Verhoeven V, Renard N, Makar A, Van Royen P, Bogers J-P, Lardon F, et al. Probiotics enhance the clearance of human papillomavirus-related cervical lesions: a prospective controlled pilot study. Eur J Cancer Prev. 2013;22:46–51. https://doi.org/10.1097/CEJ.0b013e328355ed23.
Palma E, Recine N, Domenici L, Giorgini M, Pierangeli A, Panici PB. Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: a promising solution against HPV-infection. BMC Infect Dis. 2018;18:13. https://doi.org/10.1186/s12879-017-2938-z.
Reid G, Beuerman D, Heinemann C, Bruce AW. Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunol Med Microbiol. 2001;32:37–41. https://doi.org/10.1111/j.1574-695X.2001.tb00531.x.
Morelli L, Zonenenschain D, Del Piano M, Cognein P. Utilization of the intestinal tract as a delivery system for urogenital probiotics. J Clin Gastroenterol. 2004;38:S107-110. https://doi.org/10.1097/01.mcg.0000128938.32835.98.
Homayouni A, Bastani P, Ziyadi S, Mohammad-Alizadeh-Charandabi S, Ghalibaf M, Mortazavian AM, et al. Effects of probiotics on the recurrence of bacterial vaginosis: a review. J Low Genit Tract Dis. 2014;18:79–86. https://doi.org/10.1097/LGT.0b013e31829156ec.
Liwen Z, Yu W, Liang M, Kaihong X, Baojin C. A low abundance of Bifidobacterium but not Lactobacillius in the feces of Chinese children with wheezing diseases. Medicine (Baltimore). 2018;97:e12745. https://doi.org/10.1097/MD.0000000000012745.
Onywera H, Williamson A-L, Ponomarenko J, Meiring TL. The penile microbiota in uncircumcised and circumcised men: relationships with HIV and human papillomavirus infections and cervicovaginal microbiota. Front Med. 2020;7:383. https://doi.org/10.3389/fmed.2020.00383.
Price LB, Liu CM, Johnson KE, Aziz M, Lau MK, Bowers J, et al. The effects of circumcision on the penis microbiome. PLoS ONE. 2010;5:e8422. https://doi.org/10.1371/journal.pone.0008422.
Zozaya M, Ferris MJ, Siren JD, Lillis R, Myers L, Nsuami MJ, et al. Bacterial communities in penile skin, male urethra, and vaginas of heterosexual couples with and without bacterial vaginosis. Microbiome. 2016;4:16. https://doi.org/10.1186/s40168-016-0161-6.
Brusselaers N, Shrestha S, van de Wijgert J, Verstraelen H. Vaginal dysbiosis and the risk of human papillomavirus and cervical cancer: systematic review and meta-analysis. Am J Obstet Gynecol. 2019;221(9–18):e8. https://doi.org/10.1016/j.ajog.2018.12.011.
Amador-Molina A, Hernández-Valencia JF, Lamoyi E, Contreras-Paredes A, Lizano M. Role of innate immunity against human papillomavirus (HPV) infections and effect of adjuvants in promoting specific immune response. Viruses. 2013;5:2624–42. https://doi.org/10.3390/v5112624.
Samec M, Liskova A, Koklesova L, Samuel SM, Murin R, Zubor P, et al. The role of plant-derived natural substances as immunomodulatory agents in carcinogenesis. J Cancer Res Clin Oncol. 2020;146:3137–54. https://doi.org/10.1007/s00432-020-03424-2.
Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. https://doi.org/10.1111/j.1600-065X.2008.00737.x.
Nunes RAL, Morale MG, Silva GÁF, Villa LL, Termini L. Innate immunity and HPV: friends or foes. Clinics (Sao Paulo) [Internet]. 2018 [cited 2021 Jan 29];73. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6157093/. https://doi.org/10.6061/clinics/2018/e549s.
Aristizábal B, González Á. Innate immune system [Internet]. Autoimmunity: from bench to bedside [Internet]. El Rosario University Press; 2013 [cited 2021 Jan 29]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459455/.
Ashrafian L, Sukhikh G, Kiselev V, Paltsev M, Drukh V, Kuznetsov I, et al. Double-blind randomized placebo-controlled multicenter clinical trial (phase IIa) on diindolylmethane’s efficacy and safety in the treatment of CIN: implications for cervical cancer prevention. EPMA J. 2015;6:25. https://doi.org/10.1186/s13167-015-0048-9.
Karim R, Tummers B, Meyers C, Biryukov JL, Alam S, Backendorf C, et al. Human papillomavirus (HPV) upregulates the cellular deubiquitinase UCHL1 to suppress the keratinocyte’s innate immune response. PLOS Pathogens. Public Library of Science; 2013;9 e1003384 https://doi.org/10.1371/journal.ppat.1003384
Garcia M, Alout H, Diop F, Damour A, Bengue M, Weill M, et al. Innate immune response of primary human keratinocytes to West Nile virus infection and its modulation by mosquito saliva. Front Cell Infect Microbiol [Internet]. 2018 [cited 2021 Jan 29];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6224356/. https://doi.org/10.3389/fcimb.2018.00387.
Hasan U. Human papillomavirus (HPV) deregulation of Toll-like receptor 9. Oncoimmunology [Internet]. Taylor & Francis; 2014 [cited 2021 Jan 29];3. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3935924/. https://doi.org/10.4161/onci.27257.
Hasan UA, Bates E, Takeshita F, Biliato A, Accardi R, Bouvard V, et al. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J Immunol. 2007;178:3186–97. https://doi.org/10.4049/jimmunol.178.5.3186.
Zhang Y, Yang H, Barnie PA, Yang P, Su Z, Chen J, et al. The expression of Toll-like receptor 8 and its relationship with VEGF and Bcl-2 in cervical cancer. Int J Med Sci. 2014;11:608–13. https://doi.org/10.7150/ijms.8428.
Steinbach A, Riemer AB. Immune evasion mechanisms of human papillomavirus: an update. Int J Cancer. 2018;142:224–9. https://doi.org/10.1002/ijc.31027.
Karim R, Meyers C, Backendorf C, Ludigs K, Offringa R, van Ommen GLB, et al. Human papillomavirus deregulates the response of a cellular network comprising of chemotactic and proinflammatory genes. PLoS One. 2011;6:e17848. https://doi.org/10.1371/journal.pone.0017848.
Patente TA, Pinho MP, Oliveira AA, Evangelista GCM, Bergami-Santos PC, Barbuto JAM. Human dendritic cells: their heterogeneity and clinical application potential in cancer immunotherapy. Front Immunol [Internet]. 2019 [cited 2021 Jan 29];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6348254/. https://doi.org/10.3389/fimmu.2018.03176.
Da Silva DM, Movius CA, Raff AB, Brand HE, Skeate JG, Wong MK, et al. Suppression of Langerhans cell activation is conserved amongst human papillomavirus α and β genotypes, but not a μ genotype. Virology. 2014;0:279–86. https://doi.org/10.1016/j.virol.2014.01.031.
Jimenez-Flores R, Mendez-Cruz R, Ojeda-Ortiz J, Muñoz-Molina R, Balderas-Carrillo O, de la Luz D-S, et al. High-risk human papilloma virus infection decreases the frequency of dendritic Langerhans’ cells in the human female genital tract. Immunology. 2006;117:220–8. https://doi.org/10.1111/j.1365-2567.2005.02282.x.
Su Q, Igyártó B. Keratinocytes affect biology of Langerhans cells through mRNA transfer. The Journal of Immunology American Association of Immunologists. 2018;200:109.12-109.12. https://doi.org/10.1016/j.jid.2019.05.006.
Jackson R, Eade S, Zehbe I. An epithelial organoid model with Langerhans cells for assessing virus-host interactions. Philosophical Transactions of the Royal Society B: Biological Sciences. Royal Society. 2019;374:20180288. https://doi.org/10.1098/rstb.2018.0288.
Wakabayashi R, Nakahama Y, Nguyen V, Espinoza JL. The host-microbe interplay in human papillomavirus-induced carcinogenesis. Microorganisms [Internet]. 2019 [cited 2021 Jan 30];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6680694/. https://doi.org/10.3390/microorganisms7070199.
Iijima N, Goodwin EC, Dimaio D, Iwasaki A. High-risk human papillomavirus E6 inhibits monocyte differentiation to Langerhans cells. Virology. 2013;444:257–62. https://doi.org/10.1016/j.virol.2013.06.020.
Zhou C, Tuong ZK, Frazer IH. Papillomavirus immune evasion strategies target the infected cell and the local immune system. Front Oncol [Internet]. Frontiers; 2019 [cited 2021 Jan 30];9. Available from: https://www.frontiersin.org/articles/10.3389/fonc.2019.00682/full. https://doi.org/10.3389/fonc.2019.00682.
Eiz-Vesper B, Schmetzer HM. Antigen-presenting cells: potential of proven und new players in immune therapies. Transfus Med Hemother. 2020;47:429–31. https://doi.org/10.1159/000512729.
Tran LS, Mittal D, Mattarollo SR, Frazer IH. Interleukin-17A promotes arginase-1 production and 2,4-dinitrochlorobenzene-induced acute hyperinflammation in human papillomavirus E7 oncoprotein-expressing skin. J Innate Immun. 2015;7:392–404. https://doi.org/10.1159/000374115.
Hacke K, Rincon-Orozco B, Buchwalter G, Siehler SY, Wasylyk B, Wiesmüller L, et al. Regulation of MCP-1 chemokine transcription by p53. Mol Cancer. 2010;9:82. https://doi.org/10.1186/1476-4598-9-82.
Guess JC, McCance DJ. Decreased migration of Langerhans precursor-like cells in response to human keratinocytes expressing human papillomavirus type 16 E6/E7 is related to reduced macrophage inflammatory protein-3alpha production. J Virol. 2005;79:14852–62. https://doi.org/10.1128/JVI.79.23.14852-14862.2005.
Mittal D, Kassianos AJ, Tran LS, Bergot A-S, Gosmann C, Hofmann J, et al. Indoleamine 2,3-dioxygenase activity contributes to local immune suppression in the skin expressing human papillomavirus oncoprotein e7. J Invest Dermatol. 2013;133:2686–94. https://doi.org/10.1038/jid.2013.222.
Duan S, Guo W, Xu Z, He Y, Liang C, Mo Y, et al. Natural killer group 2D receptor and its ligands in cancer immune escape. Mol Cancer [Internet]. 2019 [cited 2021 Jan 30];18. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6391774/. https://doi.org/10.1186/s12943-019-0956-8.
Jimenez-Perez MI, Jave-Suarez LF, Ortiz-Lazareno PC, Bravo-Cuellar A, Gonzalez-Ramella O, Aguilar-Lemarroy A, et al. Cervical cancer cell lines expressing NKG2D-ligands are able to down-modulate the NKG2D receptor on NKL cells with functional implications. BMC Immunol. 2012;13:7. https://doi.org/10.1186/1471-2172-13-7.
Lu J, Chatterjee M, Schmid H, Beck S, Gawaz M. CXCL14 as an emerging immune and inflammatory modulator. J Inflamm (Lond) [Internet]. 2016 [cited 2021 Jan 30];13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4700668/. https://doi.org/10.1186/s12950-015-0109-9.
Cicchini L, Westrich JA, Xu T, Vermeer DW, Berger JN, Clambey ET, et al. Suppression of antitumor immune responses by human papillomavirus through epigenetic downregulation of CXCL14. mBio. 2016;7. https://doi.org/10.1128/mBio.00270-16.
Schaerli P, Willimann K, Ebert LM, Walz A, Moser B. Cutaneous CXCL14 targets blood precursors to epidermal niches for Langerhans cell differentiation. Immunity Elsevier. 2005;23:331–42. https://doi.org/10.1016/j.immuni.2005.08.012.
Kurth I, Willimann K, Schaerli P, Hunziker T, Clark-Lewis I, Moser B. Monocyte selectivity and tissue localization suggests a role for breast and kidney–expressed chemokine (Brak) in macrophage development. J Exp Med. 2001;194:855–62. https://doi.org/10.1084/jem.194.6.855.
Yoshimura A, Muto G. TGF-β function in immune suppression. Curr Top Microbiol Immunol. 2011;350:127–47. https://doi.org/10.1007/82_2010_87.
Iancu IV, Botezatu A, Goia-Ruşanu CD, Stănescu A, Huică I, Nistor E, et al. TGF-beta signalling pathway factors in HPV-induced cervical lesions. Roum Arch Microbiol Immunol. 2010;69:113–8. ISSN 1222–3891.
Stanley MA, Sterling JC. Host responses to infection with human papillomavirus. Curr Probl Dermatol. 2014;45:58–74. https://doi.org/10.1159/000355964.
Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. 2010;125:S33-40. https://doi.org/10.1016/j.jaci.2009.09.017.
Stanley MA. Epithelial cell responses to infection with human papillomavirus. Clin Microbiol Rev. 2012;25:215–22. https://doi.org/10.1128/CMR.05028-11.
Ambagala APN, Solheim JC, Srikumaran S. Viral interference with MHC class I antigen presentation pathway: the battle continues. Vet Immunol Immunopathol. 2005;107:1–15. https://doi.org/10.1016/j.vetimm.2005.04.006.
Clark KT, Trimble CL. Current status of therapeutic HPV vaccines. Gynecol Oncol. 2020;156:503–10. https://doi.org/10.1016/j.ygyno.2019.12.017.
Beachler DC, Jenkins G, Safaeian M, Kreimer AR, Wentzensen N. Natural acquired immunity against subsequent genital human papillomavirus infection: a systematic review and meta-analysis. J Infect Dis. 2016;213:1444–54. https://doi.org/10.1093/infdis/jiv753.
Song D, Li H, Li H, Dai J. Effect of human papillomavirus infection on the immune system and its role in the course of cervical cancer (Review). Oncol Lett Spandidos Publications. 2015;10:600–6. https://doi.org/10.3892/ol.2015.3295.
Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–407. https://doi.org/10.1182/blood-2008-07-019307.
Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. Nature Publishing Group. 2007;449:819–26. https://doi.org/10.1038/nature06246.
Sato A, Iwasaki A. Induction of antiviral immunity requires Toll-like receptor signaling in both stromal and dendritic cell compartments. Proc Natl Acad Sci U S A. 2004;101:16274–9. https://doi.org/10.1073/pnas.0406268101.
Yang W, Song Y, Lu Y-L, Sun J-Z, Wang H-W. Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology. 2013;139:513–22. https://doi.org/10.1111/imm.12101.
Sheu BC, Chang WC, Lin HH, Chow SN, Huang SC. Immune concept of human papillomaviruses and related antigens in local cancer milieu of human cervical neoplasia. J Obstet Gynaecol Res. 2007;33:103–13. https://doi.org/10.1111/j.1447-0756.2007.00492.x.
Scott ME, Shvetsov YB, Thompson PJ, Hernandez BY, Zhu X, Wilkens LR, et al. Cervical cytokines and clearance of incident human papillomavirus infection: Hawaii HPV cohort study. Int J Cancer. 2013;133:1187–96. https://doi.org/10.1002/ijc.28119.
Peghini BC, Abdalla DR, Barcelos ACM, Teodoro L das GVL, Murta EFC, Michelin MA. Local cytokine profiles of patients with cervical intraepithelial and invasive neoplasia. Hum Immunol. 2012;73:920–6. https://doi.org/10.1016/j.humimm.2012.06.003.
Zhou Q, Zhu K, Cheng H. Toll-like receptors in human papillomavirus infection. Arch Immunol Ther Exp (Warsz). 2013;61:203–15. https://doi.org/10.1007/s00005-013-0220-7.
Woo YL, Sterling J, Damay I, Coleman N, Crawford R, van der Burg SH, et al. Characterising the local immune responses in cervical intraepithelial neoplasia: a cross-sectional and longitudinal analysis. BJOG. 2008;115:1616–21; discussion 1621–1622. https://doi.org/10.1111/j.1471-0528.2008.01936.x.
Jung AC, Guihard S, Krugell S, Ledrappier S, Brochot A, Dalstein V, et al. CD8-alpha T-cell infiltration in human papillomavirus-related oropharyngeal carcinoma correlates with improved patient prognosis. Int J Cancer. 2013;132:E26-36. https://doi.org/10.1002/ijc.27776.
Skeate JG, Da Silva DM, Chavez-Juan E, Anand S, Nuccitelli R, Kast WM. Nano-pulse stimulation induces immunogenic cell death in human papillomavirus-transformed tumors and initiates an adaptive immune response. PLoS ONE. 2018;13:e0191311. https://doi.org/10.1371/journal.pone.0191311.
Lee S-J, Yang A, Wu T-C, Hung C-F. Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research. J Gynecol Oncol [Internet]. 2016 [cited 2021 Feb 13];27. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4944018/. https://doi.org/10.3802/jgo.2016.27.e51.
Kovachev SM. Immunotherapy in patients with local HPV infection and high-grade squamous intraepithelial lesion following uterine cervical conization. Immunopharmacol Immunotoxicol. 2020;42:314–8. https://doi.org/10.1080/08923973.2020.1765374.
Rumfield CS, Pellom ST, Ii YMM, Schlom J, Jochems C. Immunomodulation to enhance the efficacy of an HPV therapeutic vaccine. J Immunother Cancer BMJ Specialist Journals. 2020;8:e000612. https://doi.org/10.1136/jitc-2020-000612.
Van de Wall S, Nijman HW, Daemen T. HPV-specific immunotherapy: key role for immunomodulators. Anticancer Agents Med Chem. 2014;14:265–79. https://doi.org/10.2174/187152061402140128163306.
Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9. https://doi.org/10.3390/v9090254.
Cohen AC, Roane BM, Leath CA. Novel therapeutics for recurrent cervical cancer: moving towards personalized therapy. Drugs. 2020;80:217–27. https://doi.org/10.1007/s40265-019-01249-z.
Tumer G, Simpson B, Roberts TK. Genetics, human major histocompatibility complex (MHC). StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 [cited 2021 Feb 12]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK538218/.
Mak TW, Saunders ME, Jett BD, editors. Chapter 6 - The major histocompatibility complex. Primer to the Immune Response (Second Edition) [Internet]. Boston: Academic Cell; 2014 [cited 2021 Feb 12]. p. 143–59.Available from: https://www.sciencedirect.com/science/article/pii/B9780123852458000066. ISBN 978–0–12–385245–8.
Paaso A, Jaakola A, Syrjänen S, Louvanto K. From HPV infection to lesion progression: the role of HLA alleles and host immunity. Acta Cytol. 2019;63:148–58. https://doi.org/10.1159/000494985.
Zheng Z, He Q, An L, Li D, Wang N, Wang L, et al. HLA Class II alleles and association with HPV Infection prevalence in high-risk HPV-positive Han women in southern China. Med Mal Infect. 2020. https://doi.org/10.1016/j.medmal.2020.09.006.
Gokhale P, Mania-Pramanik J, Sonawani A, Idicula-Thomas S, Kerkar S, Tongaonkar H, et al. Cervical cancer in Indian women reveals contrasting association among common sub-family of HLA class I alleles. Immunogenetics. 2014;66:683–91. https://doi.org/10.1007/s00251-014-0805-2.
Wang SS, Hildesheim A, Gao X, Schiffman M, Herrero R, Bratti MC, et al. Comprehensive analysis of human leukocyte antigen class I alleles and cervical neoplasia in 3 epidemiologic studies. J Infect Dis. 2002;186:598–605. https://doi.org/10.1086/342295.
Leo PJ, Madeleine MM, Wang S, Schwartz SM, Newell F, Pettersson-Kymmer U, et al. Defining the genetic susceptibility to cervical neoplasia—a genome-wide association study. PLOS Genetics Public Library of Science. 2017;13:e1006866. https://doi.org/10.1371/journal.pgen.1006866.
Ivansson EL, Magnusson JJ, Magnusson PKE, Erlich HA, Gyllensten UB. MHC loci affecting cervical cancer risk: distinguishing the effects of HLA-DQB1 and non-HLA genes TNF, LTA, TAP1 and TAP2. Genes Immun. 2008;9:613–23. https://doi.org/10.1038/gene.2008.58.
Chan PKS, Cheung JLK, Cheung T-H, Lin C, Tam AOY, Chan DPC, et al. HLA-B alleles, high-risk HPV infection and risk for cervical neoplasia in southern Chinese women. Int J Cancer. 2006;118:1430–5. https://doi.org/10.1002/ijc.21528.
Hu JM, Sun Q, Li L, Liu CX, Chen YZ, Zou H, et al. Human leukocyte antigen-DRB1*1501 and DQB1*0602 alleles are cervical cancer protective factors among Uighur and Han people in Xinjiang, China. Int J Clin Exp Pathol. 2014;7:6165–71.
Del Río-Ospina L, Camargo M, Soto-De León SC, Sánchez R, Moreno-Pérez DA, Patarroyo ME, et al. Identifying the HLA DRB1-DQB1 molecules and predicting epitopes associated with high-risk HPV infection clearance and redetection. Sci Rep [Internet]. 2020 [cited 2021 Feb 12];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7190668/. https://doi.org/10.1038/s41598-020-64268-x.
Bhaskaran M, Murali SV, Rajaram B, Krishnasamy S, Devasena CS, Pathak A, et al. Association of HLA-A, -B, DRB, and DQB alleles with persistent HPV-16 infection in women from Tamil Nadu. India Viral Immunol. 2019;32:430–41. https://doi.org/10.1089/vim.2019.0094.
Franciosi JR, Gelmini GF, Roxo VS, Carvalho NSde, Bicalho MdaG. Is there a role played by HLA-E, if any, in HPV immune evasion? Scand J Immunol. 2020;91:e12850. https://doi.org/10.1111/sji.12850.
Aggarwal R, Sharma M, Mangat N, Suri V, Bhatia T, Kumar P, et al. Understanding HLA-G driven journey from HPV infection to cancer cervix: adding missing pieces to the jigsaw puzzle. J Reprod Immunol. 2020;142:103205. https://doi.org/10.1016/j.jri.2020.103205.
Bubnov RV, Babenko LP, Lazarenko LM, Mokrozub VV, Spivak MY. Specific properties of probiotic strains: relevance and benefits for the host. EPMA J. 2018;9:205–23. https://doi.org/10.1007/s13167-018-0132-z.
Qingqing B, Jie Z, Songben Q, Juan C, Lei Z, Mu X. Cervicovaginal microbiota dysbiosis correlates with HPV persistent infection. Microb Pathog. 2020;104617. https://doi.org/10.1016/j.micpath.2020.104617.
Lazarenko LM, Nikitina OE, Nikitin EV, Demchenko OM, Kovtonyuk GV, Ganova LO, et al. Development of biomarker panel to predict, prevent and create treatments tailored to the persons with human papillomavirus-induced cervical precancerous lesions. EPMA J. 2014;5:1. https://doi.org/10.1186/1878-5085-5-1.
Qian S, Golubnitschaja O, Zhan X. Chronic inflammation: key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 2019;10:365–81. https://doi.org/10.1007/s13167-019-00194-x.
Łaniewski P, Cui H, Roe DJ, Barnes D, Goulder A, Monk BJ, et al. Features of the cervicovaginal microenvironment drive cancer biomarker signatures in patients across cervical carcinogenesis. Sci Rep [Internet]. 2019 [cited 2021 Jan 27];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6517407/. https://doi.org/10.1038/s41598-019-43849-5.
Łaniewski P, Barnes D, Goulder A, Cui H, Roe DJ, Chase DM, et al. Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women. Sci Rep [Internet]. 2018 [cited 2021 Jan 27];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5954126/. https://doi.org/10.1038/s41598-018-25879-7.
Hu T, Yang P, Zhu H, Chen X, Xie X, Yang M, et al. Accumulation of invariant NKT cells with increased IFN-γ production in persistent high-risk HPV-infected high-grade cervical intraepithelial neoplasia. Diagn Pathol. 2015;10:20. https://doi.org/10.1186/s13000-015-0254-8.
Gutiérrez-Hoya A, Zerecero-Carreón O, Valle-Mendiola A, Moreno-Lafont M, López-Santiago R, Weiss-Steider B, et al. Cervical cancer cells express markers associated with immunosurveillance. J Immunol Res. 2019;2019:1242979. https://doi.org/10.1155/2019/1242979.
Barros MR, de Melo CML, Barros MLCMGR, de Cássia Pereira de Lima R, de Freitas AC, Venuti A. Activities of stromal and immune cells in HPV-related cancers. Journal of Experimental & Clinical Cancer Research. 2018;37:137. https://doi.org/10.1186/s13046-018-0802-7.
Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609-616. https://doi.org/10.1016/S2214-109X(16)30143-7.
Vyshenska D, Lam KC, Shulzhenko N, Morgun A. Interplay between viruses and bacterial microbiota in cancer development. Semin Immunol. 2017;32:14–24. https://doi.org/10.1016/j.smim.2017.05.003.
Lewis FMT, Bernstein KT, Aral SO. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet Gynecol. 2017;129:643–54. https://doi.org/10.1097/AOG.0000000000001932.
Wilkinson EM, Łaniewski P, Herbst-Kralovetz MM, Brotman RM. Personal and clinical vaginal lubricants: impact on local vaginal microenvironment and implications for epithelial cell host response and barrier function. J Infect Dis. 2019;220:2009–18. https://doi.org/10.1093/infdis/jiz412.
Mändar R, Punab M, Borovkova N, Lapp E, Kiiker R, Korrovits P, et al. Complementary seminovaginal microbiome in couples. Res Microbiol. 2015;166:440–7. https://doi.org/10.1016/j.resmic.2015.03.009.
Nelson DE, Van Der Pol B, Dong Q, Revanna KV, Fan B, Easwaran S, et al. Characteristic male urine microbiomes associate with asymptomatic sexually transmitted infection. PLoS ONE. 2010;5:e14116. https://doi.org/10.1371/journal.pone.0014116.
Nelson DE, Dong Q, Van der Pol B, Toh E, Fan B, Katz BP, et al. Bacterial communities of the coronal sulcus and distal urethra of adolescent males. PLoS ONE. 2012;7:e36298. https://doi.org/10.1371/journal.pone.0036298.
Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23. https://doi.org/10.1186/s13167-016-0072-4.
Golubnitschaja O, Kinkorova J, Costigliola V. Predictive, preventive and personalised medicine as the hardcore of ‘Horizon 2020’: EPMA position paper. EPMA J. 2014;5:6. https://doi.org/10.1186/1878-5085-5-6.
Hu R, Wang X, Zhan X. Multi-parameter systematic strategies for predictive, preventive and personalised medicine in cancer. EPMA J. 2013;4:2. https://doi.org/10.1186/1878-5085-4-2.
Lu M, Zhan X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018;9:77–102. https://doi.org/10.1007/s13167-018-0128-8.
Yu JC, Khodadadi H, Baban B. Innate immunity and oral microbiome: a personalized, predictive, and preventive approach to the management of oral diseases. EPMA J. 2019;10:43–50. https://doi.org/10.1007/s13167-019-00163-4.
Funding
Open Access funding was enabled and organized by Projekt DEAL. This work was supported by the Grant Agency of the Ministry of Education of the Slovak Republic under contract no. 1/0124/17, the Slovak Research and Development Agency under contract no. APVV-16–0021, the Ministry of Health grant no. 2018/20-UKMT-16, and also by the project molecular diagnosis of cervical cancer, ITMS: 26220220113 supported by the Operational Programme Research and Innovation funded by the ERDF. D.B. was supported by a National Priorities Research Program grant (NPRP 11S-1214–170101) from the Qatar National Research Fund (QNRF, a member of Qatar Foundation).
Author information
Authors and Affiliations
Contributions
E.K. was responsible for the paper concepts, draft, and PPPM-related contents. The manuscript was drafted by E.K., A.L., M.S., L.K., V.H., T.R, E.K., and T.P.
D.B., K.Z., P.K., and K.B. critically revised the manuscript. The tables were created by A.L., M.S., and L.K. Figures were prepared by M.S. and E.K.
P.K., D.B., and K.B. provided a skilled assistance and supervised the overall preparation of the manuscript. K.Z. and D.B. were responsible for English corrections.
All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kudela, E., Liskova, A., Samec, M. et al. The interplay between the vaginal microbiome and innate immunity in the focus of predictive, preventive, and personalized medical approach to combat HPV-induced cervical cancer. EPMA Journal 12, 199–220 (2021). https://doi.org/10.1007/s13167-021-00244-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13167-021-00244-3
Keywords
- Predictive preventive and personalized medicine (PPPM/3PM)
- Gynecology
- Obstetrics
- Cancer
- HPV
- Cervical carcinogenesis
- Malignant transformation
- Cervical cancer
- Vaginal microbiome composition
- Lactobacillus
- Molecular mechanisms
- Biomarkers
- Innate immunity
- Patient stratification
- Targeted therapy
- Individualized patient profiling
- Individual outcomes