Abstract
Objective
The amplitude of low-frequency fluctuation (ALFF) fMRI technique was used to study the changes of spontaneous brain activity in patients with diabetic retinopathy and nephropathy (DRN), and to explore the application of ALFF technique in the potential prediction and the targeted prevention of diabetic microangiopathy.
Methods
Nineteen patients with diabetic retinopathy and nephropathy and 19 healthy controls (HCs) were matched for age and gender. Spontaneous cerebral activity variations were investigated using the ALFF technique. The average ALFF values of the DRN patients and the HCs were classified utilizing receiver operating characteristic (ROC) curves.
Results
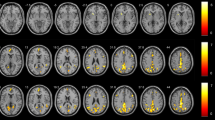
In contrast to the results in the HCs, the patients with DRN had significantly higher ALFF values in the cerebellum (bilaterally in the posterior and anterior lobes) and the left inferior temporal gyrus, but the ALFF values of the bilateral medial frontal gyrus, right superior temporal gyrus, right middle frontal gyrus, left middle/inferior frontal gyrus, bilateral precuneus, and left inferior parietal lobule were lower. ROC curve analysis of each brain region showed the accuracy of AUC was excellent. However, the mean ALFF values in the different regions did not correlate with clinical performance. The subjects showed abnormal neuronal synchronization in many areas of the brain, which is consistent with cognitive and visual functional deficits.
Conclusion
Abnormal spontaneous activity was detected in many areas of the brain, which may provide useful information for understanding the pathology of DRN. Abnormal ALFF values of these brain regions may be of predictive value in the development of early DRN and be a targeted intervention indicator for individualized treatment of diabetic microvascular diseases.





Similar content being viewed by others
References
Vaziri K, Schwartz SG, Relhan N, Kishor KS, Flynn HW. New therapeutic approaches in diabetic retinopathy. Rev Diabet Stud. 2015;12(1–2):196–210. https://doi.org/10.1900/RDS.2015.12.196.
Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614–8. https://doi.org/10.1136/bjophthalmol-2011-300539.
Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350(1):48–58 10.1056/NEJMra021678.
Takagi H. Molecular mechanisms of retinal neovascularization in diabetic retinopathy. Int Congr. 2003;4(3):299. https://doi.org/10.2169/internalmedicine.42.299.
Sagoo MK, Gnudi L. Diabetic nephropathy: is there a role for oxidative stress? Free Radic Biol Med. 2018;116:50–63. https://doi.org/10.1016/j.freeradbiomed.2017.12.040.
Patel V, Shastri M, Gaur N, Jinwala P, Kadam AY. A study in prevalence of diabetic nephropathy in recently detected cases of type 2 diabetes mellitus as evidenced by altered creatinine clearance, urinary albumin and serum creatinine, with special emphasis on hypertension, hypercholesterolemia and obesity. Int J Adv Med. 2018;5(2):351–5. https://doi.org/10.18203/2349-3933.ijam20180999.
Reutens AT. Epidemiology of diabetic kidney disease. Med Clin North Am. 2013;97(1):1–18. https://doi.org/10.1016/j.mcna.2012.10.001.
Hamat I, Abderraman GM, Cisse MM, Youssouf M, Djafar MS, Mbainguinam D, et al. Profile of diabetic nephropathy at the National Reference General Hospital of N'Djamena (Chad). Pan Afr Med J. 2016;24:193. https://doi.org/10.11604/pamj.2016.24.193.8415.
Caramori ML, Fioretto P, Mauer M. The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes. 2000;49(9):1399–408. https://doi.org/10.2337/diabetes.49.9.1399.
Saunders WB. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2):S12–154. https://doi.org/10.1053/j.ajkd.2006.12.005.
Saira C, Sumon R, Andre P, Sayon R. Tight glycemic control regulates fibronectin expression and basement membrane thickening in retinal and glomerular capillaries of diabetic rats. Invest Ophthalmol Vis Sci. 2009;50(2):943–9. https://doi.org/10.1167/iovs.08-2377.
Kramer CK, Retnakaran R. Concordance of retinopathy and nephropathy over time in type 1 diabetes: an analysis of data from the diabetes control and complications trial. Diabet Med. 2013;30(11):1333–41. https://doi.org/10.1111/dme.12296.
Barrett EJ, Liu Z, Khamaisi M, King GL, Klein R, Klein BEK, et al. Diabetic microvascular disease: an endocrine society scientific statement. J Clin Endocrinol Metab. 2017;102(12):4343–410. https://doi.org/10.1210/jc.2017-01922.
Pearce I, Simó R, Lövestam-Adrian M, Wong DT, Evans M. Association between diabetic eye disease and other complications of diabetes: implications for care. A systematic review. Diabetes Obes Metab. 2019;21(3):467–78. https://doi.org/10.1111/dom.13550.
Jeng CJ, Hsieh YT, Yang CM, Yang CH, Lin CL, Wang IJ. Diabetic retinopathy in patients with diabetic nephropathy: development and progression. PLoS One. 2016;11(8):e0161897. https://doi.org/10.1371/journal.pone.0161897.
Bekiesińska-Figatowska M, Helwich E, Rutkowska M, Stankiewicz J, Terczyńska I. Magnetic resonance imaging of neonates in the magnetic resonance compatible incubator. Arch Med Sci. 2016;12(5):1064–70. https://doi.org/10.5114/aoms.2016.61913.
Liu H, Wang X. Correlation of iron deposition and change of gliocyte metabolism in the basal ganglia region evaluated using magnetic resonance imaging techniques: an in vivo study. Arch Med Sci. 2016;12(1):163–71. https://doi.org/10.5114/aoms.2016.57593.
Dai XJ, Liu CL, Zhou RL, Gong HH, Wu B, Gao L, et al. Long-term sleep deprivation decreases the default spontaneous activity and connectivity pattern in healthy male subjects: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2015;11:761–72. https://doi.org/10.2147/NDT.S78335.
Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4(19):19. https://doi.org/10.3389/fnsys.2010.00019.
Zhang Y, Zhu C, Chen H, Duan X, Lu F, Li M, et al. Frequency-dependent alterations in the amplitude of low-frequency fluctuations in social anxiety disorder. J Affect Disord. 2015;174:329–35. https://doi.org/10.1016/j.jad.2014.12.001.
Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49(2):1432–45. https://doi.org/10.1016/j.neuroimage.2009.09.037.
Huang X, Cai FQ, Hu PH, Zhong YL, Zhang Y, Wei R, et al. Disturbed spontaneous brain- activity pattern in patients with optic neuritis using amplitude of low-frequency fluctuation: a functional magnetic resonance imaging study. Neuropsychiatr Dis Treat. 2015;11:3075–83. https://doi.org/10.2147/NDT.S92497.
Huang X, Zhong YL, Zeng XJ, Zhong YL, Zhang Y, Wei R, et al. Disturbed spontaneous brain activity pattern in patients with primary angle-closure glaucoma using amplitude of low-frequency fluctuation: a fMRI study. Neuropsychiatr Dis Treat. 2015;11:1877–83. https://doi.org/10.2147/NDT.S87596.
Tan G, Huang X, Zhang Y, Wu AH, Zhong YL, Wu K, et al. A functional MRI study of altered spontaneous brain activity pattern in patients with congenital comitant strabismus using amplitude of low-frequency fluctuation. Neuropsychiatr Dis Treat. 2016;12:1243–50. https://doi.org/10.2147/NDT.S104756.
Liang M, Xie B, Yang H, Yu L, Yin X, Wei L, et al. Distinct patterns of spontaneous brain activity between children and adults with anisometropic amblyopia: a resting-state fMRI study. Graefes Arch Clin Exp Ophthalmol. 2016;254(3):569–76. https://doi.org/10.1007/s00417-015-3117-9.
Alzner E. Bericht vom XXIX. International Congress of Ophthalmology — The World Meeting of Ophthalmologists, 21.–25. April 2002, Sydney — Australien. Spektrum Der Augenheilkunde. 2002;16(4):189–90. https://doi.org/10.1007/BF03164300.
Satterthwaite TD, Elliott MA, Gerraty RT, et al. An improved frame- work for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–56. https://doi.org/10.1016/j.neuroimage.2012.08.052.
Yan CG, Cheung B, Kelly C, Gerraty RT, Ruparel K, Loughead J, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. https://doi.org/10.1016/j.neuroimage.2013.03.004.
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–8. https://doi.org/10.1073/pnas.0504136102.
Li HJ, Dai XJ, Gong HH, Nie X, Zhang W, Peng DC. Aberrant spontaneous low-frequency brain activity in male patients with severe obstructive sleep apnea revealed by resting-state functional MRI. Neuropsychiatr Dis Treat. 2015;11:207–14. https://doi.org/10.2147/NDT.S73730.
Dai XJ, Peng DC, Gong HH, Wan AL, Nie X, Li HJ, et al. Altered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2014;10:2163–75. https://doi.org/10.2147/NDT.S69681.
Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2(1):25–32. https://doi.org/10.1089/brain.2012.0080.
Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. https://doi.org/10.1016/j.braindev.2006.07.002.
Tomino Y, Gohda T. The prevalence and management of diabetic nephropathy in Asia. Kidney Dis. 2015;1(1):52–60. https://doi.org/10.1159/000381757.
Shanbhogue VV, Hansen S, Frost M, Brixen K, Hermann AP. Bone disease in diabetes: another manifestation of microvascular disease? Lancet Diabetes Endocrinol. 2017;5(10):827–38. https://doi.org/10.1016/S2213-8587(17)30134-1.
Thapa R, Twyana SN, Paudyal G, Khanal S, van Nispen R, Tan S, et al. Prevalence and risk factors of diabetic retinopathy among an elderly population with diabetes in Nepal: the Bhaktapur Retina Study. Clin Ophthalmol. 2018;12:561–8. https://doi.org/10.2147/OPTH.S157560.
Zhao H, Ma L, Yan M, Wang Y, Zhao TT, Zhang HJ, et al. Association between MYH9 and APOL1 gene polymorphisms and the risk of diabetic kidney disease in patients with type 2 diabetes in a Chinese Han population. J Diabetes Res. 2018;1:1–6. https://doi.org/10.1155/2018/5068578.
Demerdash FE, Refaie W, Allakany R, Tantawy S, Dawood E. Diabetic retinopathy: a predictor of coronary artery disease. Egyptian Heart J. 2012;64(2):63–8. https://doi.org/10.1016/j.ehj.2011.08.006.
Bloomgarden ZT. Diabetic retinopathy and neuropathy. Diabetes Care. 2007;30(3):760. https://doi.org/10.2337/dc07-zb03.
Guo MX, Dong HH, Zhang YT, Zhang Q, Yin XH. ALFF changes in brain areas of human with high myopia revealed by resting-state functional MRI. Biomed Eng Inf. 2010;1(1):91–4. https://doi.org/10.1109/BMEI.2010.5639490.
Tan G, Huang X, Ye L, Wu AH, He LX, Zhong YL, et al. Altered spontaneous brain activity patterns in patients with unilateral acute open globe injury using amplitude of low-frequency fluctuation: a functional magnetic resonance imaging study. Neuropsychiatr Dis Treat. 2016;12(1):2015–20. https://doi.org/10.2147/NDT.S110539.
Pan ZM, Li HJ, Bao J, Jiang N, Yuan Q, Freeberg S, et al. Altered intrinsic brain activities in patients with acute eye pain using amplitude of low-frequency fluctuation: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2018;14:251–7. https://doi.org/10.2147/NDT.S150051.
Li Q, Xin H, Lei Y, Wei R, Zhang Y, Zhong YL, et al. Altered spontaneous brain activity pattern in patients with late monocular blindness in middle-age using amplitude of low-frequency fluctuation: a resting-state functional MRI study. Clin Interv Aging. 2016;11:1773–80. https://doi.org/10.2147/CIA.S117292.
Talati A, Hirsch J. Functional specialization within the medial frontal gyrus for perceptual go/no-go decisions based on “what,” “when,” and “where” related information: an fMRI study. J Cogn Neurosci. 2005;17:981–93. https://doi.org/10.1162/0898929054475226.
Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol. 1985;54:714–34. https://doi.org/10.1152/jn.1985.54.3.714.
Zald DH, McHugo M, Ray KL, Glahn DC, Eickhoff SB, Laird AR. Meta-analytic connectivity modeling reveals differential functional connectivity of the medial and lateral orbitofrontal cortex. Cereb Cortex. 2014;24(1):232–48. https://doi.org/10.1093/cercor/bhs308.
Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati P. Medial prefrontal cortex and the self in major depression. J Affect Disord. 2012;136(1–2):e1–e11. https://doi.org/10.1016/j.jad.2010.11.034.
Garcia A, Luedke A, Dowds E, Tam A, Goel A, Fernandez J. Precuneus volumes and cognitive tests in older adults. Alzheimers Dement. 2013;9(4):P795. https://doi.org/10.1016/j.jalz.2013.05.1638.
Liu Y, Li L, Li B, Feng N, Li L, Zhang X, et al. Decreased triple network connectivity in patients with recent onset post-traumatic stress disorder after a single prolonged trauma exposure. Sci Rep. 2017;7(1):12625. https://doi.org/10.1038/s41598-017-12964-6.
Vicentini JE, Weiler M, Almeida SRM, de Campos BM, Valler L, Li LM. Depression and anxiety symptoms are associated to disruption of default mode network in subacute ischemic stroke. Brain Imaging Behav. 2017;11(6):1571–80. https://doi.org/10.1007/s11682-016-9605-7.
Sieu N, Katon W, Lin EH, Russo J, Ludman E, Ciechanowski P. Depression and incident diabetic retinopathy: a prospective cohort study. Gen Hosp Psychiatry. 2011;33(5):429–35. https://doi.org/10.1016/j.genhosppsych.2011.05.021.
Themeli Y, Aliko I, Hashorva A, Bajrami V, Idrizi A, Barbullushi M, et al. P-533 - the correlation between depression and diabetic nephropathy in type 2 diabetes mellitus. Eur Psychiatry. 2012;27:1–1. https://doi.org/10.1016/S0924-9338(12)74700-4.
Wallentin M, Weed E, Østergaard L, Mouridsen K, Roepstorff A. Accessing the mental space-spatial working memory processes for language and vision overlap in precuneus. Hum Brain Mapp. 2008;29(5):524–32. https://doi.org/10.1002/hbm.20413.
Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(3):564–83. https://doi.org/10.1093/brain/awl004.
Zhang J, Su J, Wang M, Zhao Y, Yao Q, Zhang Q, et al. Increased default mode network connectivity and increased regional homogeneity in migraineurs without aura. J Headache Pain. 2016;17(1):98. https://doi.org/10.1186/s10194-016-0692-z.
Letzen JE, Robinson ME. Negative mood influences default mode network functional connectivity in patients with chronic low back pain: implications for functional neuroimaging biomarkers. Pain. 2017;158(1):48–57. https://doi.org/10.1097/j.pain.0000000000000708.
Werring DJ, Bullmore ET, Toosy AT, Miller DH, Barker GJ, MacManus DG, et al. Recovery from optic neuritis is associated with a change in the distribution of cerebral response to visual stimulation: a functional magnetic resonance imaging study. J Neurol Neurosurg Psychiatry. 2000;68(4):441–9. https://doi.org/10.1136/jnnp.68.4.441.
Wang ZL, Zou L, Lu ZW, Xie XQ, Jia ZZ, Pan CJ, et al. Abnormal spontaneous brain activity in type 2 diabetic retinopathy revealed by amplitude of low-frequency fluctuations: a resting-state fMRI study. Clin Radiol. 2017;72(4):340.e1–7. https://doi.org/10.1016/j.crad.2016.11.012.
Chyzhyk D, Graña M, Öngür D, Shinn AK. Discrimination of schizophrenia auditory hallucinators by machine learning of resting-state functional MRI. Int J Neural Syst. 2015;25(3):1550007. https://doi.org/10.1142/S0129065715500070.
Herzfeld DJ, Kojima Y, Soetedjo R, Shadmehr R. Encoding of action by the Purkinje cells of the cerebellum. Nature. 2015;526(7573):439–42. https://doi.org/10.1038/nature15693.
Timmann D, Drepper J, Frings M, Maschke M, Richter S, Gerwig M, et al. The human cerebellum contributes to motor, emotional and cognitive associative learning. A review. Cortex. 2010;46(7):845–57. https://doi.org/10.1016/j.cortex.2009.06.009.
Morenorius J. The cerebellum in fear and anxiety-related disorders. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;85:23–32. https://doi.org/10.1016/j.pnpbp.2018.04.002.
Ikeda K, Tsuchimochi H, Takeno Y, Yasuda M, Fukushima T, Toyoda K. Clinical analysis of the patients with hemodialysis associated with intracerebral hematoma. No Shinkei Geka. 2004;32(11):1133–7. https://doi.org/10.2176/nmc.44.611.
Krapfenbauer K. Identification of beta cell dysfunction at the pre-symptomatic stage of diabetes mellitus by novel analytical system: liquid biopsy measurements in femtograms. EPMA J. 2017;8(1):35–41. https://doi.org/10.1007/s13167-017-0079-5.
Duarte AA, Mohsin S, Golubnitschaja O. Diabetes care in figures: current pitfalls and future scenario. EPMA J. 2018;9(2 PG-125-131):125–31. https://doi.org/10.1007/s13167-018-0133-y.
Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7(1):1–13. https://doi.org/10.1186/s13167-016-0072-4.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
Not applicable.
Ethical approval
All the patients were informed about the purposes of the study and consequently have signed their “consent of the patient.” All investigations conformed to the principles outlined in the Declaration of Helsinki and were performed with permission by the responsible Ethics Committee of the First Affiliated Hospital of Nanchang University.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Shao, Y., Shi, WQ. et al. The predictive potential of altered spontaneous brain activity patterns in diabetic retinopathy and nephropathy. EPMA Journal 10, 249–259 (2019). https://doi.org/10.1007/s13167-019-00171-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13167-019-00171-4