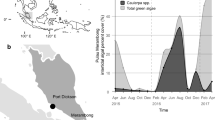
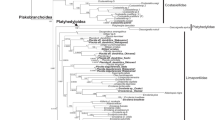
Abstract
Sacoglossa is a rather small taxon of marine slugs with about 300 described species, yet it is quite fascinating scientists for decades. This is mainly because of the ability of certain species to incorporate photosynthetically active plastids of their algae prey, a phenomenon known as functional kleptoplasty. With the stolen plastids, these slugs endure weeks (short-term retention) or months (long-term retention) of starvation, though contribution of the plastids to the survival and factors enhancing plastid longevity are unknown. Likewise, contrasting hypotheses on evolution of functional kleptoplasty exist and the phylogenetic relationship of Sacoglossa taxa is still under debate. We analyzed the phylogenetic relationship of 105 sacoglossan species to address the question of the origin of functional kleptoplasty. Based on our phylogenetic analysis and the ancestral character state reconstruction, we conclude that functional short-term retention most likely originated two times and long-term retention at least five times. Previous suggestions that functional long-term kleptoplasty is established with specific plastids are supported by our food analyses in Elysia clarki that finally harbors only plastids of certain algae species over a prolonged starvation period.



Similar content being viewed by others
References
Baumgartner, F. A., Pavia, H., & Toth, G. B. (2014). Individual specialization to non-optimal hosts in a polyphagous marine invertebrate herbivore. PLoS ONE, 9(8), e102752. doi:10.1371/journal.pone.0102752.
Bhattacharya, D., Pelletreau, K. N., Price, D. C., Sarver, K. E., & Rumpho, M. E. (2013). Genome analysis of Elysia chlorotica egg DNA provides no evidence for horizontal gene transfer into the germ line of this kleptoplastic mollusc. Molecular Biology and Evolution, 8, 1843–1852. doi:10.1093/molbev/mst084.
Christa, G., Wescott, L., Schäberle, T. F., König, G. M., & Wägele, H. (2013). What remains after 2 months of starvation? Analysis of sequestered algae in a photosynthetic slug, Plakobranchus ocellatus (Sacoglossa, Opisthobranchia), by barcoding. Planta, 237(2), 559–572. doi:10.1007/s00425-012-1788-6.
Christa, G., Zimorski, V., Woehle, C., Tielens, A. G. M., Wägele, H., Martin, W. F., & Gould, S. B. (2014a). Plastid-bearing sea slugs fix CO2 in the light but do not require photosynthesis to survive. Proceedings of the Royal Society B: Biological Sciences, 281, 20132493. doi:10.1098/rspb.2013.2493.
Christa, G., de Vries, J., Jahns, P., & Gould, S. B. (2014b). Switching off photosynthesis: the dark side of sacoglossan slugs. Communicative & Integrative Biology. doi:10.4161/cib.28029.
Christa, G., Gould, S. B., Franken, J., Vleugels, M., Karmeinski, D., Händeler, H., Martin, W.F. & Wägele, H. (2014c). Functional kleptoplasty in a limapontioid genus: phylogeny, food preferences and photosynthesis in Costasiella with focus on C. ocellifera (Gastropoda, Sacoglossa). Journal of Molluscan Studies. 1–9 doi:10.1093/mollus/eyu026.
Christa, G., Händeler, K., Schäberle, T. F., König, G. M., & Wägele, H. (2014d). Identification of sequestered chloroplasts in photosynthetic and non-photosynthetic sacoglossan sea slugs (Mollusca, Gastropoda). Frontiers in Zoology, 11, 5.
Clark, K. B., Jensen, K. R., Hugh, S. M., & Fermin, C. (1981). Chloroplast symbiosis in a non-elysiid mollusc, Costasiella lilianae (Marcus) (Hermaeidae: Ascoglossa (=Sacoglossa). Effects of temperature, light intensity, and starvation on carbon fixation rate. Biological Bulletin, 160(1), 43–54.
Clark, K. B., Jensen, K. R., & Stirts, H. M. (1990). Survey for functional kleptoplasty among west Atlantic Ascoglossa (=Sacoglossa) (Mollusca: Opisthobranchia). The Veliger, 33(4), 339–345.
Cruz, S., Calado, R., Serôdio, J., & Cartaxana, P. (2013). Crawling leaves: photosynthesis in sacoglossan sea slugs. Journal of Experimental Botany, 64, 3999–4009.
Curtis, N.E., Massey, S.E., & Pirece, S.K. (2006). The symbiotic chloroplasts in the sacoglossan Elysia clarki are from several algal species. Invertebrate Biology, 125(4), 336–345.
de Vries, J., Habicht, J., Woehle, C., Changjie, H., Christa, G., Wägele, H., et al. (2013). Is ftsH the key to plastid longevity in sacoglossan slugs? Genome Biology and Evolution, 5(12), 2540–2548. doi:10.1093/gbe/evt205.
Evertsen, J., & Johnsen, G. (2009). In vivo and in vitro differences in chloroplast functionality in the two north Atlantic sacoglossans (Gastropoda, Opisthobranchia) Placida dendritica and Elysia viridis. Marine Biology, 156(5), 847–859. doi:10.1007/s00227-009-1128-y.
Evertsen, J., Burghardt, I., Johnsen, G., & Wägele, H. (2007). Retention of functional chloroplasts in some sacoglossans from the Indo-Pacific and Mediterranean. Marine Biology, 151, 2159–2166.
Gallop, A. (1974). Evidence for the presence of a “factor” in Elysia viridis which stimulates photosynthate release from its symbiotic chloroplasts. New Phytologist, 73(6), 1111–1117.
Gallop, A., Bartrop, J., & Smith, D. C. (1980). The biology of chloroplast acquisition by Elysia viridis. Proceedings of the Royal Society B: Biological Sciences, 207(1168), 335–349. doi:10.1098/rspb.1980.0027.
Giménez-Casalduero, F., & Muniain, C. (2008). The role of kleptoplasts in the survival rates of Elysia timida (Risso, 1818): (Sacoglossa: Opisthobranchia) during periods of food shortage. Journal of Experimental Marine Biology and Ecology, 357(2), 181–187. doi:10.1016/j.jembe.2008.01.020.
Händeler, K., & Wägele, H. (2007). Preliminary study on molecular phylogeny of Sacoglossa and a compilation of their food organisms. Bonner Zoologische Beiträge, 55, 231–254.
Händeler, K., Grzymbowski, Y. P., Krug, P. J., & Wägele, H. (2009). Functional chloroplasts in metazoan cells—a unique evolutionary strategy in animal life. Frontiers in Zoology, 6(1), 28. doi:10.1186/1742-9994-6-28.
Händeler, K., Wägele, H., Wahrmund, U., Rüdinger, M., & Knoop, V. (2010). Slugs’ last meals: molecular identification of sequestered chloroplasts from different algal origins in Sacoglossa (Opisthobranchia, Gastropoda). Molecular Ecology Resources, 10, 968–978.
Hinde, R., & Smith, D. C. (1975). The role of photosynthesis in the nutrition of the mollusc Elysia viridis. Biological Journal of the Linnean Society, 7(2), 161–171. doi:10.1111/j.1095-8312.1975.tb00738.x.
Jensen, K. R. (1980). A review of sacoglossan diets, with comparative notes on radular and buccal anatomy. Malacological Review, 13, 55–77.
Jensen, K. R. (1996). Phylogenetic systematics and classification of the Sacoglossa (Mollusca, Gastropoda, Opisthobranchia). Philosophical Transactions of the Royal Society B: Biological Sciences, 351(1335), 91–122. doi:10.1098/rstb.1996.0006.
Jensen, K. R. (1997). Evolution of the Sacoglossa (Mollusca, Opisthobranchia) and the ecological associations with their food plants. Evolutionary Ecology, 11(3), 301–335.
Johnson, M. D. (2010). The acquisition of phototrophy: adaptive strategies of hosting endosymbionts and organelles. Photosynthesis Research, 107(1), 117–132. doi:10.1007/s11120-010-9546-8.
Jörger, K. M., Stöger, I., Kano, Y., Fukuda, H., Knebelsberger, T., & Schrödl, M. (2010). On the origin of Acochlidia and other enigmatic euthyneuran gastropods, with implications for the systematics of Heterobranchia. BMC Evolutionary Biology, 10(1), 323. doi:10.1186/1471-2148-10-323.
Katoh, K., Misawa, K., Kuma, K.-I., & Miyata, T. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research, 30(14), 3059–3066.
Kim, I., Rodriguez-Enriquez, S., & Lemasters, J. J. (2007). Selective degradation of mitochondria by mitophagy. Archives of Biochemistry and Biophysics, 462(2), 245–253. doi:10.1016/j.abb.2007.03.034.
Kiššová, I. B., Salin, B., Schaeffer, J., Bhatia, S., Manon, S., & Camougrand, N. (2007). Selective and non-selective autophagic degradation of mitochondria in yeast. Autophagy, 3(4), 329–336.
Klochkova, T. A., Han, J.-W., Kim, J.-H., Kim, K.-Y., & Kim, G.-H. (2010). Feeding specificity and photosynthetic activity of Korean sacoglossan mollusks. Algae, 25(4), 217–227. doi:10.4490/algae.2010.25.4.217.
Kocot, K. M., Halanych, K. M., & Krug, P. J. (2013). Phylogenomics supports Panpulmonata: Opisthobranch paraphyly and key evolutionary steps in a major radiation of gastropod molluscs. Molecular Phylogenetics and Evolution, 69(3), 764–771. doi:10.1016/j.ympev.2013.07.001.
Kohnert, P., Brenzinger, B., Jensen, K. R., & Schrödl, M. (2013). 3D-microanatomy of the semiterrestrial slug Gascoignella aprica Jensen, 1985—a basal plakobranchacean sacoglossan (Gastropoda, Panpulmonata). Organisms Diversity & Evolution, 13(4), 583–603. doi:10.1007/s13127-013-0142-6.
Kremer, B. P., & Schmitz, K. (1976). Aspects of 14CO2-fixation by endosymbiotic rhodoplasts in the marine opisthobranchiate Hermaea bifida. Marine Biology, 34(4), 313–316. doi:10.1007/BF00398124.
Krug, P. (2009). Not my “type”: larval dispersal dimorphisms and bet-hedging in opisthobranch life histories. Biological Bulletin, 216(3), 355–372.
Krug, P. J., Vendetti, J. E., Rodriguez, A. K., Retana, J. N., Hirano, Y. M., & Trowbridge, C. D. (2013). Integrative species delimitation in photosynthetic sea slugs reveals twenty candidate species in three nominal taxa studied for drug discovery, plastid symbiosis or biological control. Molecular Phylogenetics and Evolution, 69(3), 1101–1119. doi:10.1016/j.ympev.2013.07.009.
Kück, P., & Meusemann, K. (2010). FASconCAT: convenient handling of data matrices. Molecular Phylogenetics and Evolution, 56(3), 1115–1118. doi:10.1016/j.ympev.2010.04.024.
Laetz, E., Christa, G., Händeler, K., & Wägele, H. (2014). The Cylindrobulla/Ascobulla complex—unraveling problems in identification and adding to Cylindrobulla diversity by describing a new species. Zootaxa.
Lee, J., Giordano, S., & Zhang, J. (2011). Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochemical Journal, 441(2), 523–540. doi:10.1038/nature07006.
Little, D. P. (2011). DNA barcode sequence identification incorporating taxonomic hierarchy and within taxon variability. PLoS ONE, 6(8), e20552. doi:10.1371/journal.pone.0020552.s003.
Maeda, T., Kajita, T., Maruyama, T., & Hirano, Y. (2010). Molecular phylogeny of the Sacoglossa, with a discussion of gain and loss of kleptoplasty in the evolution of the group. The Biological Bulletin, 219(1), 17–26.
Maeda, T., Hirose, E., Chikaraishi, Y., Kawato, M., Takishita, K., Yoshida, T., et al. (2012). Algivore or phototroph? Plakobranchus ocellatus (Gastropoda) continuously acquires kleptoplasts and nutrition from multiple algal species in nature. PLoS ONE, 7(7), e42024. doi:10.1371/journal.pone.0042024.t004.
Marìn, A., & Ros, J. (1993). Ultrastructural and ecological aspects of the development of chloroplast retention in the sacoglossan gastropod Elysia timida. Journal of Molluscan Studies, 59(1), 95–104.
Maxwell, K., & Johnson, G. N. (2000). Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany, 51(345), 659–668.
Middlebrooks, M. L., Bell, S. S., Curtis, N. E., & Pierce, S. K. (2014). Atypical plant–herbivore association of algal food and a kleptoplastic sea slug (Elysia clarki) revealed by DNA barcoding and field surveys. Marine Biology, 161(6), 1429–1440.
Neusser, T. P., Fukuda, H., Jörger, K. M., Kano, Y., & Schrödl, M. (2011). Sacoglossa or Acochlidia? 3D reconstruction, molecular phylogeny and evolution of Aitengidae (Gastropoda: Heterobranchia). Journal of Molluscan Studies, 77(4), 332–350. doi:10.1093/mollus/eyr033.
Pagel, M., Meade, A., & Barker, D. (2004). Bayesian estimation of ancestral character states on phylogenies. Systematic Biology, 53, 673–684.
Pelletreau, K. N., Bhattacharya, D., Price, D. C., Worful, J. M., Moustafa, A., & Rumpho, M. E. (2011). Sea slug kleptoplasty and plastid maintenance in a metazoan. Plant Physiology, 155(4), 1561–1565. doi:10.1104/pp. 111.174078.
Pierce, S. K., Curtis, N. E., Massey, S. E., Bass, A. L., Karl, S. A., & Finney, C. M. (2006). A morphological and molecular comparison between Elysia crispata and a new species of kleptoplastic sacoglossan sea slug (Gastropoda: Opisthobranchia) from the Florida Keys, USA. Molluscan Research, 26(1), 23–38.
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542.
Rumpho, M. E., Summer, E. J., Green, B. J., Fox, T. C., & Manhart, J. R. (2001). Mollusc/algal chloroplast symbiosis: how can isolated chloroplasts continue to function for months in the cytosol of a sea slug in the absence of an algal nucleus? Zoology (Jena, Germany), 104(3–4), 303–312. doi:10.1078/0944-2006-00036.
Rumpho, M. E., Dastoor, F. P., Manhart, J. R., & Lee, J. (2006). The kleptoplast. In R. R. Wise & J. K. Hoober (Eds.), The structure and function of plastids (pp. 451–473). New York: Springer.
Schmitt, V., Händeler, K., Gunkel, S., Escande, M.-L., Menzel, D., Gould, S. B., et al. (2014). Chloroplast incorporation and long-term photosynthetic performance through the life cycle in laboratory cultures of Elysia timida (Sacoglossa, Heterobranchia). Frontiers in Zoology, 11(1), 5. doi:10.1186/1742-9994-11-5.
Serôdio, J., Cruz, S., Cartaxana, P., & Caldo, R. (2014). Photophysiology of kleptoplasts: photosynthetic use of light by chloroplasts living in animal cells. Philosophical Transactions of the Royal Society B: Biological Science, 369, 20130242. doi:10.1098/rstb.2013.0242.
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22(21), 2688–2690. doi:10.1093/bioinformatics/btl446.
Taylor, D. L. (1971). Symbiosis between the chloroplasts of Griffithsia flosculosa (Rhodophyta) and Hermaea bifida (Gastropoda: Opisthobranchia). Pubblicazioni della Stazione Zoologica di Napoli, 39, 116–120.
Trench, R. K. (1973). Further studies on the mucopolysaccharide secreted by the pedal gland of the marine slug Tridachia crispata. Bulletin of Marine Science, 23, 299–312.
Trench, R. K. (1975). Of “leaves that crawl”: functional chloroplasts in animal cells. Symposia of the Society for Experimental Biology, 29, 229–265.
Trench, R. K., & Gooday, G. W. (1973). Incorporation of [H-3]-leucine into protein by animal tissues and by endosymbiotic chloroplasts in Elysia viridis Montagu. Comparative Biochemistry and Physiology, 44(2A), 321–330.
Wägele, H., & Martin, W. F. (2013). In W. Löffelhardt (Ed.), Endosymbioses in sacoglossan seaslugs: plastid-bearing animals that keep photosynthetic organelles without borrowing genes (pp. 291–324). Vienna: Endosymbiosis. doi:10.1007/978-3-7091-1303-5_14.
Wägele, H., Deusch, O., Händeler, K., Martin, R., Schmitt, V., Christa, G., et al. (2011). Transcriptomic evidence that longevity of acquired plastids in the photosynthetic slugs Elysia timida and Plakobranchus ocellatus does not entail lateral transfer of algal nuclear genes. Molecular Biology and Evolution, 28(1), 699–706. doi:10.1093/molbev/msq239.
Wägele, H., Klussmann-Kolb, A., Verbeek, E., & Schrödl, M. (2014). Flashback and foreshadowing—a review of the taxon Opisthobranchia. Organisms, Diversity & Evolution, 14, 133–149.
Waugh, G. R., & Clark, K. B. (1986). Seasonal and geographic variation in chlorophyll level of Elysia tuca (Ascoglossa: Opisthobranchia). Marine Biology, 92(4), 483–487. doi:10.1007/BF00392508.
Wright, S. W., & Grant, B. R. (1978). Properties of chloroplasts isolated from siphonous algae: effects of osmotic shock and detergent treatment on intactness. Plant Physiology, 61(5), 768–771.
Yamamoto, Y. Y., Yusa, Y., Yamamoto, S., Hirano, Y., Hirano, Y., Motomura, T., et al. (2009). Identification of photosynthetic sacoglossans from Japan. Endocytobiosis and Cell Research, 19, 112–119.
Yamamoto, S., Hirano, Y. M., Hirano, Y. J., Trowbridge, C. D., Akimoto, A., Sakai, A., & Yusa, Y. (2013). Effects of photosynthesis on the survival and weight retention of two kleptoplastic sacoglossan opisthobranchs. Journal of the Marine Biological Association of the United Kingdom, 93(01), 209–215. doi:10.1017/S0025315412000628.
Acknowledgments
This project was funded by a grant given to HW (Wa 618/8 and Wa 618/12). We thank the staff of the Mote Marine Laboratory, of the Keys Marine Laboratory, and of Lizard Island Research Station for their assistance. We thank Valerie Schmitt and Jan de Vries for helping to collect the specimens. We thank two anonymous reviewers for their comments helping to improve the manuscript. The material was legally collected and exported according to the Australian und US laws.
Authors’ contribution
GC, KH, and HW planned the experiments and collected the material. GC, HW, DK, JF, MV, and PK performed the experiments and analyzed the molecular data. GC, DK, JF, MV, PK, and HW analyzed the data and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Food sources of Sacoglossan slugs identified by DNA barcoding in this study (PDF 898 kb)
Supplementary Fig. 2
Food sources of non-starved Elysia clarki identified by DNA barcoding in this study (PDF 84 kb)
Supplementary Fig. 3
Food sources of starved Elysia clarki identified by DNA barcoding in this study (PDF 90 kb)
Supplementary Fig. 4
Network analysis on COI sequences for species identification of sacoglossan specimen used for food analysis (PDF 24 kb)
Supplementary Table 1
Ancestral character state reconstruction of functional kleptoplasty performed with BayesTraits (PDF 18 kb)
Supplementary Data 1
List of species used for phylogenetic analysis (PDF 208 kb)
Supplementary Data 2
Food sources and PAM measurements of sacoglossan sea slugs (PDF 4160 kb)
Rights and permissions
About this article
Cite this article
Christa, G., Händeler, K., Kück, P. et al. Phylogenetic evidence for multiple independent origins of functional kleptoplasty in Sacoglossa (Heterobranchia, Gastropoda). Org Divers Evol 15, 23–36 (2015). https://doi.org/10.1007/s13127-014-0189-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-014-0189-z