Abstract
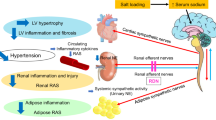
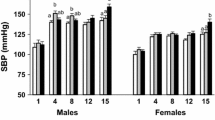
The mechanisms involved in renal dysfunction induced by high-fat diet (HFD) in subjects with altered renal development (ARDev) are understudied. The objective of this study is to examine whether there are sex-dependent differences in the mechanisms involved in the hypertension and deterioration of renal function in SD rats with prolonged HFD and ARDev. The role of angiotensin II (Ang II) in the arterial pressure (AP) increments, the renal hemodynamic sensitivity to Ang II, glomerular damage and changes in fat abdominal volume, plasma adipokine levels, renal NADPHp67phox expression, and renal infiltration of immune cells were examined. Hypertension and deterioration of renal function were enhanced (P < 0.05) in both sexes of rats with HFD and ARDev. The decrease (P < 0.05) of AP elicited by candesartan in hypertensive rats was similar to that induced by the simultaneous administration of candesartan and apocynin. The greater (P < 0.05) renal vasoconstriction induced by Ang II in both sexes of rats with HFD and ARDev was accompanied by an enhanced (P < 0.05) infiltration of CD-3 cells and macrophages in the renal cortex and renal medulla. The increments (P < 0.05) in the renal expression of NADPHp67phox and glomeruloesclerosis were greater (P < 0.05) in males than in females with HFD and ARDev. Our results suggest that the hypertension and deterioration of renal function induced by HFD in rats with ARDev are Ang II-dependent and mediated by increments in oxidative stress and immune system activation. Sex-dependent increments in oxidative stress and glomerular damage may contribute to the deterioration of renal function in these rats.







Similar content being viewed by others
References
Adler S, Huang H (2004) Oxidant stress in kidneys of spontaneously hypertensive rats involves both oxidase overexpression and loss of extracellular superoxide dismutase. Am J Physiol Ren Physiol 287:F907–F913. https://doi.org/10.1152/ajprenal.00060.2004
Agarwal R (2003) Proinflammatory effects of oxidative stress in chronic kidney disease: role of additional angiotensin II blockade. Am J Physiol Ren Physiol 284:F863–F869. https://doi.org/10.1152/ajprenal.00385.2002
Bouloumie A, Marumo T, Lafontan M, Busse R (1999) Leptin induces oxidative stress in human endothelial cells. FASEB J 13:1231–1238
Dalmassso C, Leachman JR, Ensor CM, Yiannikouris FB, Giani JF, Cassis LA, Loria AS (2019) Female mice exposed to postnatal neglect display angiotensin II-dependent obesity-induced hypertension. J Am Heart Assoc 8:e012309. https://doi.org/10.1161/JAHA.119.012309
De Miguel C, Guo C, Lund H, Feng D, Mattson DL (2011) Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Ren Physiol 300:F734–F742. https://doi.org/10.1152/ajprenal.00454.2010
Fernandes R, Garver H, Harkema JR, Galligan JJ, Fink GD, Xu H (2018) Sex differences in renal inflammation and injury in high-fat diet-fed Dahl salt-sensitive rats. Hypertension 72:e43–e52. https://doi.org/10.1161/HYPERTENSIONAHA.118.11485
Gilbert JS, Nijland MJ (2008) Sex differences in the developmental origins of hypertension and cardiorenal disease. Am J Phys Regul Integr Comp Phys 295:R1941–R1952. https://doi.org/10.1152/ajpregu.90724.2008
Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME (2015) Obesity-induced hypertension: interaction of neurohormonal and renal mechanisms. Circ Res 116:991–1006. https://doi.org/10.1161/CIRCRESAHA.116.305697
Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh H, Weyand CM (2011) Inflammation, immunity and hypertension. Hypertension 57:132–140. https://doi.org/10.1161/HYPERTENSIONAHA.110.163576
Hsu CN, Lai WT, Lin YJ, Tain YL (2018) Postnatal high-fat diet sex-specifically exacerbates prenatal dexamethasone-induced hypertension. Mass spectrometry-based quantitative proteomic approach. J Nutr Biochem 57:268–275. https://doi.org/10.1016/j.jnutbio.2018.04.006
Imig JD, Ryan MJ (2013) Immune and inflammatory role in renal disease. Compr Physiol 3:957–976. https://doi.org/10.1002/cphy.c120028
Intapad S, Dasinger JH, Johnson JM, Brown AD, Ojeda NB, Alexander NT (2019) Male and female intrauterine growth-restricted offspring differ in blood pressure, renal function, and glucose homeostasis responses to a postnatal diet high in fat and sugar. Hypertension 73:620–629. https://doi.org/10.1161/HYPERTENSIONAHA.118.12134
Ji HJ, Zheng W, Li X, Liu J, Wu X, Zhang MA, Umans JG, Hay M, Speth RC, Dunn SE, Sandberg K (2014) Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension 64:573–582. https://doi.org/10.1161/HYPERTENSIONAHA.114.03663
Knight SF, Yuan J, Roy S, Imig JD (2010) Simvastatin and tempol protect against endothelial dysfunction and renal injury in a model of obesity and hypertension. Am J Physiol Ren Physiol 298:F86–F94. https://doi.org/10.1152/ajprenal.00351.2009
Lee MYK, Ge G, Fung ML, Vanhoutte PM, Mak JCW, Ip MSM (2018) Low but not high frequency of intermittent hypoxia suppresses endothelium-dependent, oxidative stress-mediated contractions in carotid arteries of obese mice. J Appl Physiol 125:1384–1395. https://doi.org/10.1152/japplphysiol.00224.2018
Lin YJ, Lin IC, Yu HR, Sheen JM, Huang LT, Tain YL (2018) Early postweaning treatment with dimethyl fumarate prevents prenatal dexamethasone- and postnatal high-fat-induced programmed hypertension in male rat offspring. Oxidative Med Cell Longev 2018:5343462–5343468. https://doi.org/10.1155/2018/5343462
Moreno JM, Tapia A, Martinez CM, Reverte V, Oltra L, Llinas MT, Salazar FJ (2019) Sex-dependent differences in the adverse renal changes induced by an early in life exposure to a high-fat diet. Am J Physiol Ren Physiol 316:F332–F340. https://doi.org/10.1152/ajprenal.00394.2018
Murphy MO, Herald JB, Wills CT, Unfried SG, Cohn DM, Loria AS (2017) Postnatal treatment with metyrapone attenuates the effects of diet-induced obesity in female rats exposed to early-life stress. Am J Physiol Endocrinol Metab 312:E98–E108. https://doi.org/10.1152/ajpendo.00308.2016
Nogueira A, Pires MJ, Oliveira PA (2017) Pathophysiological mechanisms of renal fibrosis: a review of animal models and therapeutical strategies. In Vivo 31:1–22. https://doi.org/10.21873/invivo.11019
Obata Y, Yamada Y, Takashi Y, Baden MY, Saisho K, Tamba S, Yamamoto K, Umeda M, Furubayashi A, Matsuzawa Y (2013) Relationship between serum adiponectin levels and age in healthy subjects and patients with type 2 diabetes. Clin Endocrinol 79:204–210. https://doi.org/10.1111/cen.12041
Paquete K, Fernandes RO, Xie F, Cloutier A, Fallaha C, Girard-Bock C, Rehman MO, Mian R, Lukaszewky MA, Mässe B, El-Jabout R, Lapeyraque AL, Santos RA, Luu TM, Nuyt AM (2018) Kidney size, renal function, angiotensin peptide, and blood pressure in young adults born preterm. The HAPI study. Hypertension 72:918–928. https://doi.org/10.1161/HYPERTENSIONAHA.118.11397
Pedone C, Roshanravan B, Scariata S, Patel KV, Ferrucci L, Incalzi RA (2015) Longitudinal association between serum leptin concentration and glomerular filtration rate in humans. PLoS ONE 10:e0117828. https://doi.org/10.1371/journal.pone.0117828
Pollow DP, Uhrlaub J, Romero-Aleshire MJ, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M (2014) Sex-differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension 64:384–390. https://doi.org/10.1161/HYPERTENSIONAHA.114.03581
Praga M (2005) Synergy of low nephron number and obesity: a new focus on hyperfiltration nephropathy. Nephrol Dial Transplant 20:2594–2597. https://doi.org/10.1093/ndt/gfi201
Reverte V, Tapia A, Moreno JM, Rodriguez L, Salazar F, Llinas MT, Salazar FJ (2011) Renal effects of prolonged high protein intake and COX2 inhibition on hypertensive rats with altered renal development. Am J Physiol Ren Physiol 301:F327–F333. https://doi.org/10.1152/ajprenal.00110.2011
Reverte V, Tapia A, Baile G, Gambini J, Gimenez I, Llinas MT, Salazar FJ (2013) Role of angiotensin II on arterial pressure and renal hemodynamics in rats with an altered renal development. Age- and sex-dependent differences. Am J Physiol Ren Physiol 304:F33–F40. https://doi.org/10.1152/ajprenal.00424.2012
Saez F, Castell MT, Zuasti A, Salazar F, Reverte V, Loria A, Salazar FJ (2007) Sex differences in the renal changes elicited by angiotensin II blockade during the nephrogenic period. Hypertension 49:1429–1435. https://doi.org/10.1161/HYPERTENSIONAHA.107.087957
Saez F, Reverte V, Paliege A, Moreno JM, Linás MT, Bachmann S, Salazar FJ (2014) Sex-dependent hypertension and renal changes in aged rats with altered renal development. Am J Physiol Ren Physiol 307:F461–F470. https://doi.org/10.1152/ajprenal.00198.2014
Salazar FJ, Reverte V, Saez F, Loria A, Llinas MT, Salazar FJ (2008) Age- and sodium-sensitive hypertension and sex-dependent renal changes in rats with a reduced nephron number. Hypertension 51:1184–1189. https://doi.org/10.1161/HYPERTENSIONAHA.107.100750
Savino A, Pelliccia P, Chiarelli F, Mohn A (2010) Obesity-related renal injury in childhood. Horm Res Paediatr 73:303–311. https://doi.org/10.1159/000308161
Tomat AL, Salazar FJ (2014) Mechanisms involved in developmental programming of hypertension and renal diseases. Gender differences. Horm Mol Biol Clin Invest 18:63–77. https://doi.org/10.1515/hmbci-2013-0054
Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ (2008) Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int 73:19–33. https://doi.org/10.1038/sj.ki.5002586
Williams PJ, Kurlak LO, Perkins AC, Budge H, Stephenson T, Keisler D, Symonds ME, Gardner DS (2007) Hypertension and impaired renal function accompany juvenile obesity: the effect of prenatal diet. Kidney Int 72:279–289. https://doi.org/10.1038/sj.ki.5002276
Availability of data and material
All raw data that support the findings of this study will be available without restriction from the corresponding author on reasonable request.
Funding
This work was supported by the Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación (PI16/01556) (co-funded by European Regional Development Fund/European Social Fund “A way to make Europe/Investing in your future) and Fondos FEDER.
Author information
Authors and Affiliations
Contributions
- Moreno JM, Salazar FJ, and Llinas MT contributed to the conception and design of the work.
- All authors contributed to the acquisition, analysis, or interpretation of data and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
The procuration of animals, the husbandry, and the experiments conform to the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes. Approval was obtained from the ethics committee of the University of Murcia, Spain.
Consent for publication
All authors revised the manuscript and approved the version to be published.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
F. Javier Salazar and María T. Llinás share senior authorship.
Key Points
• An early and prolonged high-fat diet induces a greater increment of arterial pressure in male than in female rats with a reduced nephron endowment.
• Renal damage is enhanced by a high-fat diet in both sexes of rats with altered renal development.
• Angiotensin II is involved in the hypertension and deterioration of renal function in rats with a prolonged high-fat diet and reduced nephron endowment.
• The activation of oxidative stress and inflammatory pathways is greater in male than in female rats with a prolonged high-fat diet and reduced nephron endowment.
Rights and permissions
About this article
Cite this article
Moreno, J.M., Martinez, C.M., de Jodar, C. et al. Gender differences in the renal changes induced by a prolonged high-fat diet in rats with altered renal development. J Physiol Biochem 77, 431–441 (2021). https://doi.org/10.1007/s13105-021-00815-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-021-00815-y