Abstract
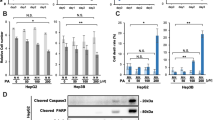
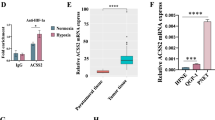
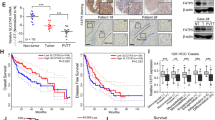
Lipid metabolism rewiring in gastric adenocarcinoma (GA) pathogenesis is still not clearly elucidated. This study aimed to describe the role of lipid catabolism in GA patient outcomes and possible therapeutic targets by analyzing the effect of hypoxia-inducible factor-1α (HIF-1α) on fatty acid oxidation (FAO). AGS cell line was cultured in normoxic and hypoxic conditions, and FAO-related genes were analyzed by real-time-PCR and Western-blot. The study group comprised 108 newly diagnosed GA patients and 152 control cases. Serum concentrations of medium and long-chain acyl-CoA dehydrogenases (MCAD and LCAD) proteins were measured using ELISA, and local expression of HIF-1α, carnitine palmitoyl transferase 1 (CPT1A) and peroxisome proliferator-activated receptor γ (PPARγ) was evaluated by immunohistochemistry. In addition, gene expression of PPARγ, CPT1A, LCAD, and MCAD was assessed by real-time-PCR. In vitro findings indicate HIF-1α upregulation and FAO-related genes and proteins reduction in the hypoxic culture of AGS cells. GA patients had significantly lower circulating levels of LCAD compared to controls. Higher protein expression of HIF-1α and downregulated CPT1A and PPARγ were observed in GA tissues versus controls. Gene expression of CPT1A, PPARγ, LCAD, and MCAD were repressed in GA tissues compared to controls. Moreover, reduced expression of CPT1A, PPARγ, and MCAD were correlated with HIF-1α upregulation in GA. Poor patient outcome was associated with lower PPARγ and LCAD expression in GA. HIF-1α upregulation in human GA patients and AGS cells was paralleled by downregulation of lipid catabolism genes potentially via reduced PPARγ-mediated FAO. This metabolic adaptation to hypoxic condition may play a role in GA pathogenesis and might have clinical and therapeutic value in GA patients.






Similar content being viewed by others
Data availability
The data are available from the corresponding author upon request.
References
Aiderus A, Black MA, Dunbier AK (2018) Fatty acid oxidation is associated with proliferation and prognosis in breast and other cancers. BMC Cancer 18:805
Bensaad K, Favaro E, Lewis CA, Peck B, Lord S, Collins JM, Pinnick KE, Wigfield S, Buffa FM, Li J-L (2014) Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep 9:349–365
Bernardo BC, Weeks KL, Pongsukwechkul T, Gao X, Kiriazis H, Cemerlang N, Boey EJ, Tham YK, Johnson CJ, Qian H (2018) Gene delivery of medium chain acyl-coenzyme A dehydrogenase induces physiological cardiac hypertrophy and protects against pathological remodelling. Clin Sci 132:381–397
Boström P, Br M, Svensson P-A, Wiklund O, Borén J, Carlsson LM, Ståhlman M, Olofsson S-O, Hultén LM (2006) Hypoxia converts human macrophages into triglyceride-loaded foam cells. Arterioscler Thromb Vasc Biol 26:1871–1876
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 348:1625–1638
Cascone T, McKenzie JA, Mbofung RM, Punt S, Wang Z, Xu C, Williams LJ, Wang Z, Bristow CA, Carugo A (2018) Increased tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metab 27:977–987.e974
Du W, Zhang L, Brett-Morris A, Aguila B, Kerner J, Hoppel CL, Puchowicz M, Serra D, Herrero L, Rini BI (2017) HIF drives lipid deposition and cancer in ccRCC via repression of fatty acid metabolism. Nat Commun 8:1769
Enjoji M, Kohjima M, Ohtsu K, Matsunaga K, Murata Y, Nakamuta M, Imamura K, Tanabe H, Iwashita A, Nagahama T (2016) Intracellular mechanisms underlying lipid accumulation (white opaque substance) in gastric epithelial neoplasms: a pilot study of expression profiles of lipid-metabolism-associated genes. J Gastroenterol Hepatol 31:776–781
Ezzeddini R, Taghikhani M, Somi MH, Samadi N, Rasaee MJ (2019) Clinical importance of FASN in relation to HIF-1α and SREBP-1c in gastric adenocarcinoma. Life Sci 224:169–176. https://doi.org/10.1016/j.lfs.2019.03.056
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Forootan FS, Forootan SS, Malki MI, Chen D, Li G, Lin K, Rudland PS, Foster CS, Ke Y (2014) The expression of C-FABP and PPARγ and their prognostic significance in prostate cancer. Int J Oncol 44:265–275
Gordon GB, Barcza MA, Bush ME (1977) Lipid accumulation in hypoxic tissue culture cells. Am J Pathol 88:663
Huang D, Li T, Li X, Zhang L, Sun L, He X, Zhong X, Jia D, Song L, Semenza GL (2014) HIF-1-mediated suppression of acyl-CoA dehydrogenases and fatty acid oxidation is critical for cancer progression. Cell Rep 8:1930–1942
Huang M, Koizumi A, Narita S, Inoue T, Tsuchiya N, Nakanishi H, Numakura K, Tsuruta H, Saito M, Satoh S (2016) Diet-induced alteration of fatty acid synthase in prostate cancer progression. Oncogenesis 5:e195–e195
Kurtz DM, Rinaldo P, Rhead WJ, Tian L, Millington DS, Vockley J, Hamm DA, Brix AE, Lindsey JR, Pinkert CA (1998) Targeted disruption of mouse long-chain acyl-CoA dehydrogenase gene reveals crucial roles for fatty acid oxidation. Proc Natl Acad Sci U S A 95:15592–15597
Lauren P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma: an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64:31–49
Lee HJ, Ryu JM, Jung YH, Oh SY, Lee SJ, Han HJ (2015) Novel pathway for hypoxia-induced proliferation and migration in human mesenchymal stem cells: involvement of HIF-1α, FASN, and mTORC1. Stem Cells 33:2182–2195
Lewis C, Brault C, Peck B, Bensaad K, Griffiths B, Mitter R, Chakravarty P, East P, Dankworth B, Alibhai D (2015) SREBP maintains lipid biosynthesis and viability of cancer cells under lipid-and oxygen-deprived conditions and defines a gene signature associated with poor survival in glioblastoma multiforme. Oncogene 34:5128–5140
Liu Y, Ma Z, Zhao C, Wang Y, Wu G, Xiao J, McClain CJ, Li X, Feng W (2014) HIF-1α and HIF-2α are critically involved in hypoxia-induced lipid accumulation in hepatocytes through reducing PGC-1α-mediated fatty acid β-oxidation. Toxicol Lett 226:117–123
Lu J, Imamura K, Nomura S, K-i M, Nakajima A, Kadowaki T, Kubota N, Terauchi Y, Ishii G, Ochiai A (2005) Chemopreventive effect of peroxisome proliferator–activated receptor γ on gastric carcinogenesis in mice. Cancer Res 65:4769–4774
Lu X, Bijli KM, Ramirez A, Murphy TC, Kleinhenz J, Hart CM (2013) Hypoxia downregulates PPARγ via an ERK1/2–NF-κB–Nox4-dependent mechanism in human pulmonary artery smooth muscle cells. Free Radic Biol Med 63:151–160
Ma Y, Temkin SM, Hawkridge AM, Guo C, Wang W, Wang X-Y, Fang X (2018) Fatty acid oxidation: an emerging facet of metabolic transformation in cancer. Cancer Lett 435:92–100
Mousavi SM, Gouya MM, Ramazani R, Davanlou M, Hajsadeghi N, Seddighi Z (2008) Cancer incidence and mortality in Iran. Ann Oncol 20:556–563
Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM (2010) Rosiglitazone attenuates chronic hypoxia–induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol 42:482–490
Norris AW, Chen L, Fisher SJ, Szanto I, Ristow M, Jozsi AC, Hirshman MF, Rosen ED, Goodyear LJ, Gonzalez FJ (2003) Muscle-specific PPARγ-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest 112:608–618
Ogino S, Shima K, Baba Y, Nosho K, Irahara N, Kure S, Chen L, Toyoda S, Kirkner GJ, Wang YL (2009) Colorectal cancer expression of peroxisome proliferator-activated receptor γ (PPARG, PPARgamma) is associated with good prognosis. Gastroenterology 136:1242–1250
Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC (2006) HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 3:187–197
Pope ED III, Kimbrough EO, Vemireddy LP, Surapaneni PK, Copland JA III, Mody K (2019) Aberrant lipid metabolism as a therapeutic target in liver cancer. Expert Opin Ther Targets 23(6):473–483
Riester M, Xu Q, Moreira A, Zheng J, Michor F, Downey R (2017) The Warburg effect: persistence of stem-cell metabolism in cancers as a failure of differentiation. Ann Oncol 29:264–270
Seyfried TN, Flores RE, Poff AM, D’agostino DP (2013) Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis 35:515–527
Srivastava N, Kollipara RK, Singh DK, Sudderth J, Hu Z, Nguyen H, Wang S, Humphries CG, Carstens R, Huffman KE (2014) Inhibition of cancer cell proliferation by PPARγ is mediated by a metabolic switch that increases reactive oxygen species levels. Cell Metab 20:650–661
Stephens NA, Skipworth RJ, MacDonald AJ, Greig CA, Ross JA, Fearon KC (2011) Intramyocellular lipid droplets increase with progression of cachexia in cancer patients. J Cachexia Sarcopenia Muscle 2:111–117
Sumi C, Okamoto A, Tanaka H, Kusunoki M, Shoji T, Uba T, Adachi T, Iwai T, Nishi K, Harada H (2018) Suppression of mitochondrial oxygen metabolism mediated by the transcription factor HIF-1 alleviates propofol-induced cell toxicity. Sci Rep 8:8987
Tan CK, Zhuang Y, Wahli W (2017) Synthetic and natural peroxisome proliferator-activated receptor (PPAR) agonists as candidates for the therapy of the metabolic syndrome. Expert Opin Ther Targets 21:333–348
Tolwani RJ, Hamm DA, Tian L, Sharer JD, Vockley J, Rinaldo P, Matern D, Schoeb TR, Wood PA (2005) Medium-chain acyl-CoA dehydrogenase deficiency in gene-targeted mice. PLoS Genet 1:e23
Tsubaki M, Takeda T, Tomonari Y, Kawashima K, Itoh T, Imano M, Satou T, Nishida S (2018) Pioglitazone inhibits cancer cell growth through STAT3 inhibition and enhanced AIF expression via a PPARγ-independent pathway. J Cell Physiol 233:3638–3647
Vayalil PK, Landar A (2015) Mitochondrial oncobioenergetic index: a potential biomarker to predict progression from indolent to aggressive prostate cancer. Oncotarget 6:43065–43080
Wada S, Yasunaga Y, Oka K, Dan N, Tanaka E, Morita K, Masuda E, Yanagawa K, Matsumoto H, Yoshioka S (2018) Submucosal fat accumulation in human colorectal tissue and its association with abdominal obesity and insulin resistance. United European Gastroenterol J 6:1065–1073
Whitmer JT, Idell-Wenger JA, Rovetto MJ, Neely JR (1978) Control of fatty acid metabolism in ischemic and hypoxic hearts. J Biol Chem 253:4305–4309
Woo CC, Loo SY, Gee V, Yap CW, Sethi G, Kumar AP, Tan KHB (2011) Anticancer activity of thymoquinone in breast cancer cells: possible involvement of PPAR-γ pathway. Biochem Pharmacol 82:464–475
Youssef J, Badr M (2011) Peroxisome proliferator-activated receptors and cancer: challenges and opportunities. Br J Pharmacol 164:68–82
Zeng W, Liu P, Pan W, Singh SR, Wei Y (2015) Hypoxia and hypoxia inducible factors in tumor metabolism. Cancer Lett 356:263–267
Zhang K, Han ES, Dellinger TH, Lu J, Nam S, Anderson RA, Yim JH, Wen W (2017) Cinnamon extract reduces VEGF expression via suppressing HIF-1α gene expression and inhibits tumor growth in mice. Mol Carcinog 56:436–446
Zhao M, Li X, Zhang Y, Zhu H, Han Z, Kang Y (2020) PPARG drives molecular networks as an inhibitor for the pathologic development and progression of lung adenocarcinoma. PPAR Res 2020:1–7
Acknowledgements
The authors are grateful to Professor Kh. Adeli and V. Higgins from Toronto University for valuable scientific comments on the manuscript. We thank Dr. Amirtaher Eftekharosadat from Liver and Gastrointestinal Diseases Research Center of Tabriz for evaluating the pathological sections. Our thanks also to the staff on the endoscopy ward from Liver and Gastrointestinal Diseases Research Center of Tabriz for kind cooperation. Great supports from Professor SM. Moazzeni are appreciated.
Funding
This work was supported by Iran National Science Foundation (Grant number: 94016621), Tarbiat Modares University (Grant number: 52D.7928), Liver and Gastrointestinal Diseases Research Center (Grant number: 144.257), and Hematology and Oncology Research Center (Grant number: 95.1).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: R.E.; M.T.;. M.H.S.; N.S. Performed the experiments: R.E.; A.S.F. Analyzed and interpreted the data: R.E.; M.T.; M.J.R.; A.S.F.; A.E. Contributed reagents/materials/analysis tools: R.E.; M.T.;. M.H.S.; A.S.F.; N.S. Wrote the paper: R.E.; A.S.F.; M.J.R; A.E. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The Human Research Ethics Committees of Tarbiat Modares University (Tehran, Iran) (Ethic code: IR.TMU.REC.1394.184) and Hematology and Oncology Research Center (Tabriz, Iran) (Ethic code: TBZMED.REC.1395.12) approved the study protocols. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
HIF-1α-mediated metabolic adaptation in human GA is correlated with downregulation of FAO genes via reduced PPARγ.
FAO metabolic repression seems to be involved in GA pathogenesis and is associated with poor patient outcome.
Rights and permissions
About this article
Cite this article
Ezzeddini, R., Taghikhani, M., Salek Farrokhi, A. et al. Downregulation of fatty acid oxidation by involvement of HIF-1α and PPARγ in human gastric adenocarcinoma and related clinical significance. J Physiol Biochem 77, 249–260 (2021). https://doi.org/10.1007/s13105-021-00791-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-021-00791-3