Abstract
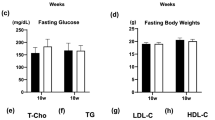
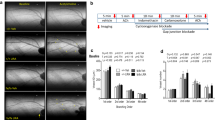
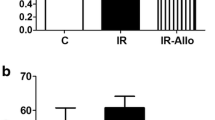
Obesity is the major risk factor for metabolic syndrome and atherosclerotic cardiocerebrovascular diseases and induces insulin resistance characterized by a dysfunction of insulin to activate insulin receptor /insulin receptor substrate 1(IRS-1)/phosphoinositide 3-kinase (PI3K)/Akt pathway. Zucker fatty rats (8 weeks) were treated with vehicle (0.5 % methyl cellulose in physiological saline, p.o.), amlodipine (3 mg/kg/day, p.o.), atorvastatin (10 mg/kg/day, p.o.), or the combination of amlodipine plus atorvastatin (3 + 10 mg/kg/day, p.o.) for 28 days, and anti-insulin-like growth factor 1 (IGF-1)/IRS-1/PI3K/Akt pathways were evaluated. Our present immunohistochemical study first demonstrated that a combination of amlodipine plus atorvastatin treatment prevented an arteriosclerotic process compared to the single treatment with amlodipine or atorvastatin with strong recoveries of pTyr IRS-1, pPI3K, and pAkt expressions and with remarkable restraints of IGF-1 and pSer IRS-1. As a result, combination therapy with amlodipine plus atorvastatin showed a strong synergistic effect to prevent atherosclerotic processes. The present study newly suggests a synergistic benefit of combination therapy with amlodipine plus atorvastatin for strong prevention of atherosclerotic processes, which could reduce the clinical risk of cerebrovascular events for obesity patients.





Similar content being viewed by others
Abbreviations
- IGF-1:
-
Insulin-like growth factors 1
- IRS-1:
-
Insulin receptor substrate 1
- PI3K:
-
Phosphoinositide 3-kinase
References
Braunersreuther V, Mach F, Steffens S. The specific role of chemokines in atherosclerosis. Thromb Haemost. 2007;97(5):714–21.
Salminen A, Kaarniranta K. Insulin/IGF-1 paradox of aging: regulation via AKT/IKK/NF-kappaB signaling. Cell Signal. 2010;22(4):573-7.
Kramsch DM, Sharma RC. Limits of lipid-lowering therapy: the benefits of amlodipine as an anti-atherosclerotic agent. J Hum Hypertens. 1995;9 Suppl 1:S3–9.
Thaulow E, Jorgensen B. Results and clinical implications of the CAPARES trial. Can J Cardiol. 2000;16(Suppl D):8D–11.
Li XQ, Cao W, Li T, Zeng AG, Hao LL, Zhang XN, et al. Amlodipine inhibits TNF-alpha production and attenuates cardiac dysfunction induced by lipopolysaccharide involving PI3K/Akt pathway. Int Immunopharmacol. 2009;9(9):1032–41.
Bell RM, Yellon DM. Atorvastatin, administered at the onset of reperfusion, and independent of lipid lowering, protects the myocardium by up-regulating a pro-survival pathway. J Am Coll Cardiol. 2003;41(3):508–15.
Zhang L, Zhang ZG, Liu XS, Hozeska-Solgot A, Chopp M. The PI3K/Akt pathway mediates the neuroprotective effect of atorvastatin in extending thrombolytic therapy after embolic stroke in the rat. Arterioscler Thromb Vasc Biol. 2007;27(11):2470–5.
Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–58.
Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–96.
Nissen SE, Tuzcu EM, Libby P, Thompson PD, Ghali M, Garza D, et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA. 2004;292(18):2217–25.
Vaziri ND, Xu ZG, Shahkarami A, Huang KT, Rodriguez-Iturbe B, Natarajan R. Role of AT-1 receptor in regulation of vascular MCP-1, IL-6, PAI-1, MAP kinase, and matrix expressions in obesity. Kidney Int. 2005;68(6):2787–93.
Fulton D, Harris MB, Kemp BE, Venema RC, Marrero MB, Stepp DW. Insulin resistance does not diminish eNOS expression, phosphorylation, or binding to HSP-90. Am J Physiol Heart Circ Physiol. 2004;287(6):H2384–93.
Zucker LM, Antoniades HN. Insulin and obesity in the Zucker genetically obese rat “fatty”. Endocrinology. 1972;90(5):1320–30.
Zucker LM. Hereditary obesity in the rat associated with hyperlipemia. Ann N Y Acad Sci. 1965;131(1):447–58.
Contreras C, Sanchez A, Garcia-Sacristan A, Martinez MC, Andriantsitohaina R, Prieto D. Preserved insulin vasorelaxation and up-regulation of the Akt/eNOS pathway in coronary arteries from insulin resistant obese Zucker rats. Atherosclerosis. 2011;217(2):331-9.
Osmond JM, Mintz JD, Stepp DW. Preventing increased blood pressure in the obese Zucker rat improves severity of stroke. Am J Physiol Heart Circ Physiol. 2010;299(1):H55-61.
Laight DW, Kengatharan KM, Gopaul NK, Anggard EE, Carrier MJ. Investigation of oxidant stress and vasodepression to glyceryl trinitrate in the obese Zucker rat in vivo. Br J Pharmacol. 1998;125(4):895–901.
Andrews TJ, Laight DW, Anggard EE, Carrier MJ. Investigation of endothelial hyperreactivity in the obese Zucker rat in-situ: reversal by vitamin E. J Pharm Pharmacol. 2000;52(1):83–6.
Subramanian R, MacLeod KM. Age-dependent changes in blood pressure and arterial reactivity in obese Zucker rats. Eur J Pharmacol. 2003;477(2):143–52.
Dorafshar AH, Moodley K, Khoe M, Lyon C, Bryer-Ash M. Pioglitazone improves superoxide dismutase mediated vascular reactivity in the obese Zucker rat. Diab Vasc Dis Res. 2010;7(1):20-7.
Katakam PV, Snipes JA, Steed MM, Busija DW. Insulin-induced generation of reactive oxygen species and uncoupling of nitric oxide synthase underlie the cerebrovascular insulin resistance in obese rats. J Cereb Blood Flow Metab. 2012;32(5):792–804.
Katakam PV, Snipes JA, Tulbert CD, Mayanagi K, Miller AW, Busija DW. Impaired endothelin-induced vasoconstriction in coronary arteries of Zucker obese rats is associated with uncoupling of [Ca2+]i signaling. Am J Physiol Regul Integr Comp Physiol. 2006;290(1):R145–53.
Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, et al. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104(4):447–57.
Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105(3):311–20.
Howard G, O'Leary DH, Zaccaro D, Haffner S, Rewers M, Hamman R, et al. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation. 1996;93(10):1809–17.
Ruige JB, Assendelft WJ, Dekker JM, Kostense PJ, Heine RJ, Bouter LM. Insulin and risk of cardiovascular disease: a meta-analysis. Circulation. 1998;97(10):996–1001.
Kobayashi T, Kaneda A, Kamata K. Possible involvement of IGF-1 receptor and IGF-binding protein in insulin-induced enhancement of noradrenaline response in diabetic rat aorta. Br J Pharmacol. 2003;140(2):285–94.
Kobayashi T, Matsumoto T, Kamata K. IGF-I-induced enhancement of contractile response in organ-cultured aortae from diabetic rats is mediated by sustained thromboxane A2 release from endothelial cells. J Endocrinol. 2005;186(2):367–76.
Kanda T, Wakino S, Homma K, Yoshioka K, Tatematsu S, Hasegawa K, et al. Rho-kinase as a molecular target for insulin resistance and hypertension. FASEB J. 2006;20(1):169–71.
Zick Y. Role of Ser/Thr kinases in the uncoupling of insulin signaling. Int J Obes Relat Metab Disord. 2003;27 Suppl 3:S56–60.
Anai M, Funaki M, Ogihara T, Terasaki J, Inukai K, Katagiri H, et al. Altered expression levels and impaired steps in the pathway to phosphatidylinositol 3-kinase activation via insulin receptor substrates 1 and 2 in Zucker fatty rats. Diabetes. 1998;47(1):13–23.
Hamamdzic D, Fenning RS, Patel D, Mohler ER, 3rd, Orlova KA, Wright AC, et al. Akt pathway is hypoactivated by synergistic actions of diabetes mellitus and hypercholesterolemia resulting in advanced coronary artery disease. Am J Physiol Heart Circ Physiol. 2010;299(3):H699-706.
Kobayashi T, Taguchi K, Yasuhiro T, Matsumoto T, Kamata K. Impairment of PI3-K/Akt pathway underlies attenuated endothelial function in aorta of type 2 diabetic mouse model. Hypertension. 2004;44(6):956–62.
Annoura H, Nakanishi K, Uesugi M, Fukunaga A, Miyajima A, Tamura-Horikawa Y, et al. A novel class of Na+ and Ca2+ channel dual blockers with highly potent anti-ischemic effects. Bioorg Med Chem Lett. 1999;9(20):2999–3002.
Loke KE, Messina EJ, Mital S, Hintze TH. Impaired nitric oxide modulation of myocardial oxygen consumption in genetically cardiomyopathic hamsters. J Mol Cell Cardiol. 2000;32(12):2299–306.
Lukic-Panin V, Kamiya T, Zhang H, Hayashi T, Tsuchiya A, Sehara Y, et al. Prevention of neuronal damage by calcium channel blockers with antioxidative effects after transient focal ischemia in rats. Brain Res. 2007;1176:143–50.
Rami A, Langhagen A, Steiger S. Focal cerebral ischemia induces upregulation of Beclin 1 and autophagy-like cell death. Neurobiol Dis. 2008;29(1):132–41.
Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6(9):1004–10.
Nagotani S, Hayashi T, Sato K, Zhang W, Deguchi K, Nagano I, et al. Reduction of cerebral infarction in stroke-prone spontaneously hypertensive rats by statins associated with amelioration of oxidative stress. Stroke. 2005;36(3):670–2.
Tsuchiya A, Nagotani S, Hayashi T, Deguchi K, Sehara Y, Yamashita T, et al. Macrophage infiltration, lectin-like oxidized-LDL receptor-1, and monocyte chemoattractant protein-1 are reduced by chronic HMG-CoA reductase inhibition. Curr Neurovasc Res. 2007;4(4):268–73.
Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Lund DD, Yorek MA. Attenuation of vascular/neural dysfunction in Zucker rats treated with enalapril or rosuvastatin. Obesity (Silver Spring). 2008;16(1):82–9.
Oltman CL, Kleinschmidt TL, Davidson EP, Coppey LJ, Lund DD, Yorek MA. Treatment of cardiovascular dysfunction associated with the metabolic syndrome and type 2 diabetes. Vascul Pharmacol. 2008;48(1):47–53.
Kawai H, Deguchi S, Deguchi K, Yamashita T, Ohta Y, Omote Y, et al. Protection against ischemic stroke damage by synergistic treatment with amlodipine plus atorvastatin in Zucker metabolic rat. Brain Res. 2011;1382:308-14.
Kawai H, Deguchi S, Deguchi K, Yamashita T, Ohta Y, Shang J, et al. Synergistic benefit of combined amlodipine plus atorvastatin on neuronal damage after stroke in Zucker metabolic rat. Brain Res. 2011;1368:317-23.
Acknowledgments
This study was supported in part by Grant-in-Aid for Scientific Research (B) 21390267 and the Ministry of Education, Science, Culture and Sports of Japan; by Grants-in-Aid from the Research Committee of CNS Degenerative Diseases (Nakano I); and by grants (Mizusawa H, Nishizawa M, Sasaki H, and Sobue G) from the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawai, H., Tian, F., Kurata, T. et al. Prevention of Hyperglycemic Signal Pathways in Metabolic Syndrome Carotid Artery of Rats. Transl. Stroke Res. 3, 466–472 (2012). https://doi.org/10.1007/s12975-012-0205-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-012-0205-6