Abstract
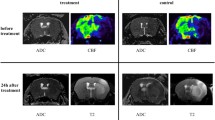
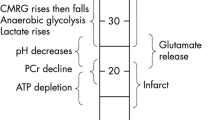
The ischemic penumbra is the target of acute stroke therapy. It can be approximated on diffusion/perfusion MRI as the ischemic region with abnormal perfusion and normal diffusion imaging. Using arterial spin labeling perfusion MRI and diffusion MRI, our group has studied the evolution of the diffusion/perfusion mismatch in rat stroke models. Additionally, we have evaluated the effects of high-flow oxygen on the natural history of penumbral evolution, demonstrating that high-flow oxygen “freezes” the evolution of the mismatch and allows for later beneficial use of intravenous tissue plasminogen activator (tPA). Two neuroprotective drugs, Granulocyte colony stimulating factor and a PSD95 inhibitor both impeded the evolution of the mismatch into infarcted tissue in vivo and by histological analysis. Employing a novel technique of clot imaging, our group was able to demonstrate that the combination of tPA plus Annexin-2 was superior to tPA alone in dissolving an embolus and also in reducing the extent of hypoperfused brain tissue of perfusion imaging. The use of these advanced MRI techniques in animal experiments will help to advance clinical imaging of the ischemic penumbra and hopefully contribute to the extension of the therapeutic time window in stroke patients.




Similar content being viewed by others
References
Lo EH, Moskowitz MA, Jacobs TP. Exciting, radical, suicidal: how brain cells die after stroke. Stroke. 2005;36:189–92.
Martini SR, Kent TA. Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab. 2007;27:435–51.
Chalela J, Kidwell C, Nentwich L. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293–8.
Liu KF, Li F, Tatlisumak T, Garcia JH, Sotak CH, Fisher M, et al. Regional variations in the apparent diffusion coefficient and the intracellular distribution of water in rat brain during acute focal ischemia. Stroke. 2001;32:1897–905.
Heiss W. Ischemic penumbra: evidence from functional imaging in man. J Cereb Blood Flow Metab. 2000;20:1276–93.
Baird AS, Warach SW. Magnetic resonance imaging of acute stroke. J Cereb Blood Flow Metab. 1998;18:583–609.
Detre JA, Alsop DC, Vives LR, Maccotta L, Teener JW, Raps EC. Noninvasive MRI evaluation of cerebral blood flow in cerebrovascular disease. Neurology. 1998;50:633–41.
Wintermark M, Sesay M, Barbier E, et al. Comparative overview of brain perfusion imaging techniques. Stroke. 2005;36:e83–9.
Calamante F, Thomas DL, Pell GS, Wiersma J, Turner R. Measuring cerebral blood flow using magnetic resonance imaging techniques. J Cereb Blood Flow Metab. 1999;19:701–35.
Schlaug G, Benfield A, Baird AE, et al. The ischemic penumbra operationally defined by diffusion-perfusion MRI. Neurology. 1999;53:1528–37.
Kidwell CS, Saver JL, Mattiello J, et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol. 2000;47:462–9.
Kane I, Carpenter T, Chappell F, et al. Comparison of 10 different magnetic resonance perfusion imaging processing methods in acute ischemic stroke. Stroke. 2007;38:3158–64.
Olivot JM, Miynash M, Thijs VN, et al. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke. 2009;40:469–75.
Meng X, Fisher M, Shen Q, Sotak CH, Duong TQ. Characterizing the diffusion/perfusion mismatch in a rat stroke model of focal cerebral ischemia. Ann Neurol. 2004;55:207–12.
Bardutzky J, Shen Q, Ren H, Duong TQ, Fisher M. Differences in ischemic lesion evolution in different rat strains using diffusion and perfusion imaging. Stroke. 2005;36:2000–5.
Henninger N, Sicard KM, Schmidt K, Bardutzky J, Fisher M. Comparison of ischemic lesion evolution in embolic vs. mechanical middle cerebral artery occlusion in Sprague–Dawley rats using diffusion and perfusion imaging. Stroke. 2006;37:1283–7.
Shen Q, Ren H, Fisher M, Duong TQ. Statistical prediction of tissue fate in acute in ischemic brain injury. JCBFM. 2005;25:1336–45.
Henninger N, Bouley J, Nelligan J, Sicard KM, Fisher M. Normobaric hyperoxia delays perfusion/diffusion mismatch evolution, reduces infarct volume and differentially affects neuronal cell death pathways after suture middle cerebral artery occlusion in rats. JCBFM. 2007;27:1632–42.
Henninger N, Bratane BT, Bastan B, Bouley J, Fisher M. Normobaric hyperoxia and late tPA in a rat embolic stroke model. J Cereb Blood Flow Metab. 2009;29:119–29.
Bratane B, Bouley J, Schneider A, Bastan B, Henninger N, Fisher M. Granulocyte colony stimulating factor delays mismatch evolution and reduces final infarct volume in permanent suture and embolic rat focal cerebral ischemia models. Stroke. 2009;40:3102–6.
Bratane B, Cui H, Cook DJ, Bouley J, Tymianski M, Fisher M. Neuroprotection by freezing ischemic penumbra evolution with PSD95 inhibitor. Stroke (in press).
Walvick RP, Bratane B, Henninger N, et al. Evaluation of clot lysis in a rat embolic stroke model. Stroke. 2011;42:1110–5.
Fisher M, Bastan B. Identifying and utilizing the ischemic penumbra. Neurology (in press).
Potential Conflict of Interest
The G-CSF study was partially funded by Sygnis Bioscience and the PSD95 Inhibitor study was partially funded by the Chair fund of the Neurovascular Therapeutics Program from the University Health Network, Toronto.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fisher, M., Bråtane, B.T. Imaging of Experimental Stroke Models. Transl. Stroke Res. 3, 16–21 (2012). https://doi.org/10.1007/s12975-011-0113-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-011-0113-1