Abstract
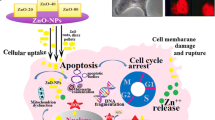
Zinc oxide nanoparticles are presently being used in cosmetics, paints, biosensors, and medical applications. However, zinc oxide nanoparticles are broadly used in the cosmetic industry for many years; recently, they have been explored for use in cancer therapy. This study was intended to comprehend the mechanism of the differential cytotoxicity of rod and spherical shaped zinc oxide nanoparticles to cervical cancer cells HeLa and SiHa. The role of ion dissolution in the toxicity of zinc oxide nanoparticles was also investigated. Zinc oxide nanorod induced significantly higher oxidative stress, reactive oxygen species compared to spherical shaped zinc oxide nanoparticles. Additionally, rod shaped nanoparticles activated the NF-κB signaling pathway. Besides, zinc oxide nanorod also induced mitochondrial membrane damage leading to apoptosis as observed by PARP cleavage and reduction of Phospho-Bad. In conclusion, zinc oxide nanorod, after proper tailoring, may have potential to use as chemotherapeutic agents against cervical cancer.










Similar content being viewed by others
Change history
20 August 2019
The original article unfortunately contains minor errors in Fig. 1B.
20 August 2019
The original article unfortunately contains minor errors in Fig. 1B.
Abbreviations
- NPs:
-
Nanoparticles
- ZnO NPs:
-
Zinc oxide nanoparticles
- ROS:
-
Reactive oxygen species
- MMP:
-
Mitochondrial membrane potential
- TEM:
-
Transmission electron microscope
- FE-SEM:
-
Field-emission scanning electron microscope
- DLS:
-
Dynamic light scattering
- PDI:
-
Polydispersity index
- XRD:
-
X-ray diffraction
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- DCFDA:
-
2,7-Dichlorofluorescein di-acetate
- MDA:
-
Malondialdehyde
- LPO:
-
Lipid peroxidation
- GSH:
-
Glutathione
- PC:
-
Protein carbonyl
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- LDH:
-
Lactate dehydrogenase
- JC-1 dye:
-
5,5′,6,6′-Tetrachloro-1,1′,3,3-tetraethylbenzimidazolcarbocyanine iodide
- NF-κB:
-
Nuclear factor-kappa beta
- PARP:
-
Poly ADP ribose polymerase
- ARS:
-
Alizarin Red S
- OD:
-
Optical density.
References
Serpone, N., Dondi, D., Albini, A. (2007). Inorganic and organic UV filters: their role and efficiency in sunscreens and suncare products. Inorganica Chim Acta, 306, 794–803.
Yuranova, T., Laub, D., Kiwi, J. (2007). Synthesis, activity and characterization of textiles showing self-cleaning activity under daylight irradiation. Catalysis Today, 122, 109–117.
Wang, B., Feng, W., Wang, M., Wang, T., Gu, Y., Zhu, M., et al. (2008). Acute toxicological impact of nano-and sub micro-scaled zinc oxide powder on healthy adult mice. Journal of Nanoparticle Research, 10, 263–276.
Hanley, C., Layne, J., Punnoose, A., Reddy, K. M., Coombs, I., Coombs, A., et al. (2008). Preferential killing of cancer cells and activated human T cells using zinc oxide nanoparticles. Nanotechnology, 19, 295103.
Premanathan, M., Karthikeyan, K., Jeyasubramanian, K., Manivannan, G. (2011). Selective toxicity of ZnO nanoparticles toward Gram positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine, 7, 184–192.
Jia, H. Y., Liu, Y., Zhang, X. J., Han, L., Du, L. B., Tian, Q., et al. (2008). Potential oxidative stress of gold nanoparticles by induced-NO releasing in serum. Journal of the American Chemical Society, 131, 14261–14263.
Durocher, S., Rezaee, A., Hamm, C., Rangan, C., Mittler, S., Mutus, B. (2009). Disulfide linked, gold nanoparticle based teagent for detecting small molecular weight thiols. Journal of the American Chemical Society, 131, 2475–2477.
Xia, T., Kovochich, M., Liong, M., Madler, L., Gilbert, B., Shi, H. (2008). Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano, 2, 2121–2134.
Heng, B. C., Zhao, X., Xiong, S., Ng, K. W., Boey, F. Y. C., Loo, J. S. C. (2011). Cytotoxicity of zinc oxide (ZnO) nanoparticles is influenced by cell density and culture format. Archives of Toxicology, 85, 695–704.
Sharma, V., Shukla, R. K., Saxena, N., Parmar, D., Das, M., Dhawan, A. (2009). DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicology Letters, 185, 211–218.
Lin, W. S., Xu, Y., Huang, C. C., Ma, Y. F., Shannon, K. B., Chen, D. R., et al. (2009). Toxicity of nano- and micro-sized ZnO particles in human lung epithelial cells. Journal of Nanoparticle Research, 11, 25–39.
Sharma, V., Anderson, D., Dhawan, A. (2012). Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis, 17, 852–870.
Hsiao, I. L., & Huang, Y. J. (2011). Effects of various physicochemical characteristics on the toxicities of ZnO and TiO2 nanoparticles toward human lung epithelial cells. Science of the Total Environment, 409, 1219–1228.
Kang, T., Guan, R., Chen, X., Song, Y., Jiang, H., Zhao, J. (2013). In vitro toxicity of different-sized ZnO nanoparticles in Caco-2 cells. Nanoscale Research Letters, 8, 496–501.
Chhabra, S., Bhavani, M., Mahajan, N., Bawaskar, R. (2010). Cervical cancer in Indian rural women: trends over two decades. Journal of Obstetrics and Gynaecology, 30, 725–728.
Yuan, J. H., Chen, Y., Zha, H. X., Song, H. J., Li, C. Y., Li, J., et al. (2010). Determination, characterization and cytotoxicity on human embryonic lung fibroblast of ZnO nanoparticles. Colloids and Surfaces B: Biointerfaces, 76, 145–150.
Huang, C. C., Aronstam, R. S., Chen, D. R., Huang, Y. W. (2010). Oxidative stress, calcium homeostasis, and altered gene expression in human lung epithelial cells exposed to ZnO nanoparticles. Toxicology In Vitro, 24, 45–55.
Wahab, R., Kaushik, N. K., Verma, A. K., Mishra, A., Hwang, I. H., Yang, Y. B., et al. (2011). Fabrication and growth mechanism of ZnO nanostructures and their cytotoxic effect on human brain tumor U87, cervical cancer HeLa, and normal HEK cells. Journal of Biological Inorganic Chemistry, 16, 431–42.
Bhattacharya, D., Santra, C. R., Ghosh, A. N., Karmakar, P. (2014). Differential toxicity of rod and spherical zinc oxide nanoparticles on human peripheral blood mononuclear cells. Journal of Biomedical Nanotechnology, 10, 707–716.
Bhattacharya, D., Samanta, S., Mukherjee, A., Santra, C. R., Ghosh, A. N., Karmakar, P. (2012). Antibacterial activities of polyethylene glycol, tween 80 and sodium dodecyl sulphate coated silver nanoparticles in normal and multi-drug resistant bacteria. Journal of Nanoscience and Nanotechnology, 12, 2513–2521.
Shamsi, F. A., & Boulton, M. (2001). Inhibition of RPE lysosomal and antioxidant activity by the age pigment lipofuscin. Investigative Ophthalmology & Visual Science, 42, 3041–3046.
Levine, R. L., Garland, D., Oliver, C. N., Amici, A., Climent, I., Lenz, A. G., et al. (1990). Determination of carbonyl contents of oxidatively modified proteins. Methods in Enzymology, 186, 464–478.
Kakkar, P. S., Das, B., Viswanathan, P. N. (1984). A modified spectrophotometric assay of superoxide dismutase. Indian Journal of Biochemistry & Biophysics, 21, 130–132.
Sinha, A. K. (1972). Colorimetric assay of catalase. Analytical Biochemistry, 47, 389–394.
Ohkawa, H., Ohishi, N., Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 95, 351–358.
Vassault, A. (1983) Lactate dehydrogenase. In H. Bergemeyer (Ed.), Methods of Enzyme Analysis (p. 118). Weinheim: Verlag Chemie.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Bagchi, B., Kar, S., Dey, S., Bhandary, S., Roy, D., Mukhopadhyay, T., et al. (2013). In situ synthesis and antibacterial activity of copper nanoparticle loaded natural montmorillonite clay based on contact inhibition and ion release. Colloids and Surfaces B: Biointerfaces, 108, 358–365.
Jeng, H. A., & Swanson, J. (2006). Toxicity of metal oxide nanoparticles on mammalian cells. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 41, 2699–2711.
Hu, X. K., Cook, S., Wang, P., Hwang, H. M. (2009). In vitro evaluation of cytotoxicity of engineered metal oxide nanoparticles. Science of the Total Environment, 407, 3070–3072.
Yang, S. T., Liu, J. H., Wang, J., Yuan, Y., Cao, A. N., Wang, H. F., et al. (2010). Cytotoxicity of zinc oxide nanoparticles: importance of microenvironment. Journal of Nanoscience and Nanotechnology, 10, 8638–8645.
Xia, T., Kovochich, M., Brant, J., Hotze, M., Sempf, J., Oberley, T., et al. (2006). Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Letters, 6, 1794–1797.
Bishop, G. M., Dringen, R., Robinson, S. R. (2007). Zinc stimulates the production of toxic reactive oxygen species (ROS) and inhibits glutathione reductase in astrocytes. Free Radical Biology & Medicine, 42, 1222–1230.
Ahamed, M., Akhtar, M. J., Raja, M., Ahmad, I., Siddiqui, M. K. J., AlSalhi, M. S., et al. (2011). Zinc oxide nanorod induced apoptosis via p53, bax/bcl-2 and survivin pathways in human lung cancer cells: role of oxidative stress. Nanomedicine, 7, 904–913.
Akhtar, M. J., Ahamed, M., Kumar, S., Khan, M. A. M., Ahmed, J., Alrokayan, S. A. (2012). ZnO nanorod-induced apoptosis in human alveolar adenocarcinoma cells via p53, survivin and bax/bcl-2 pathways: role of oxidative stress. International Journal of Nanomedicine, 7, 845–857.
Wang, J. J., Sanderson, J. S. B., Wang, H. (2007). Cyto- and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutation Research, 628, 99–106.
Møller, P., Jacobsen, N. R., Folkmann, J. K., Danielsen, P. H., Mikkelsen, L., Hemmingsen, J. G., et al. (2010). Role of oxidative damage in toxicity of particulates. Free Radical Research, 44, 1–46.
Karin, M., & Lin, A. (2002). NF-κB at the crossroads of life and death. Nature Immunology, 3, 221–227.
Reuter, S., Eifes, S., Dicato, M., Aggarwal, B. B., Diederich, M. (2008). Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochemical Pharmacology, 76, 1340–1351.
Elmore, S. (2007). Apoptosis: a review of programmed cell death. Toxicologic Pathology, 35, 495–516.
Perchellet, E. M., Wang, Y., Weber, R. L., Sperfslage, B. J., Lou, K., Crossland, J., et al. (2004). Synthetic 1,4-anthracenedione analogs induce cytochrome C release, caspase-9, -3 and -8 activities, poly(ADP-ribose) polymerase-1 cleavage and internucleosomal DNA fragmentation in HL-60 cells by a mechanism which involves caspase-2 activation but not Fas signaling. Biochemical Pharmacology, 67, 523–537.
Laha, D. R., Bhattacharya, D., Pramanik, A., Santra, C. R., Pramanik, P., Karmakar, P. (2012). Evaluation of copper iodide and copper phosphate nanoparticles for their potential cytotoxic effect. Toxicology Research, 1, 131–136.
Brunner, T. J., Wick, P., Manser, P., Spohn, P., Grass, P. N., Limbach, L., et al. (2006). In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environmental Science & Technology, 40, 4374–4381.
Acknowledgments
This work was financially supported by UPE (Phase II), DST-PURSE program and State Government Fellowship scheme of Jadavpur University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. The authors alone are responsible for the content and writing of the article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1585 kb)
Rights and permissions
About this article
Cite this article
Bhattacharya, D., Bhattacharyya, A. & Karmakar, P. Evaluation of Different Oxidative Stress Parameters and Apoptosis in Human Cervical Cancer Cells Exposed to Rod and Spherical Shaped Zinc Oxide Nanoparticles. BioNanoSci. 6, 1–14 (2016). https://doi.org/10.1007/s12668-015-0186-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-015-0186-5