Abstract
A non-equiatomic Fe34.9Ni30.2Mn18.6Cr9.3Al7 high-entropy alloy (HEA) was synthesized by mechanical alloying using different milling times. To study the effect of milling time on the structure stability, X-ray diffraction (XRD), scanning electron microscopy and energy-dispersive X-ray spectroscopy were conducted. For comparison, an Al-free alloy (Fe37.5Ni32.5Mn20Cr10) was produced at 25 h milling. XRD indicated a single FCC phase alloy after 5 h milling time and a dual FCC and BCC phase at 25 h milling time. Clearly, it is found that Al addition is not necessarily the main factor leading to BCC phase formation as reported for similar HEAs produced by casting route. Increasing the milling time, the lattice strain increased reaching an average maximum value of 0.8% with an increase in d-spacing while the crystallite size was reduced to about 5.7 nm. A dual-phase structure formation was related to a decrease in the accumulated strain (ca.32%) confirming a strain-induced transformation.
Similar content being viewed by others
1 Introduction
The continuing demand for alloys with superior properties has driven the search for new materials with enhanced stability. A wide area of current research is the development of non-equiatomic high-entropy alloys (HEAs). Cantor et al and Yeh et al were the first to use the term "High-Entropy Alloys" in 2004 when multi-principal elements alloys were first produced at equiatomic proportions with other elements [1, 2]. HEAs mostly contain at least five or more principal elements maximum up to thirteen, each having an atomic percentage between 5 and 35%. The atomic percentage of each minor element, if any, is hence less than 5% [3].
HEAs are produced using manufacturing techniques similar to conventional materials such as liquid melting, solid-state mechanical alloying (MA), and gas-state mixing [3]. The produced phases depend on the processing route used for alloy fabrication. MA is considered an important solid-state fabrication method to synthesize both equilibrium and non-equilibrium phases of commercially useful and scientifically interesting materials [4].
MA has been the most popular technique for pre-alloyed powders because it allows obtaining almost any composition possible with quite enough time and energy [5]. It is also used for alloys containing low or high melting point elements, synthesizing nanocrystalline materials with consistent microstructures and compositional homogeneity [6]. The development of a fine mixture of secondary phase particles, extending the limits of solid solubility, reducing grain sizes to the nanometer range, synthesis of new crystalline and quasi-crystalline structures, forming amorphous phases, and promoting chemical reactions at low temperatures are some key features of MA [7]. However, forming homogeneous compounds through MA depends on different processing factors. These include the milling time, rotational speed, the process control agent, atmospheric conditions, and ball to powder weight ratio [5].
HEAs produced by MA have shown promising technological aspects in many applications such as hard coatings on various substrate materials [8, 9]. Ball-milled AlCoCrFeNiSi HEA powder with dual-phase structure was successfully synthesized and then plasma sprayed on 316 stainless steel substrates [10]. CoCrFeNi and CoCrFeNiCu HEA coatings were also prepared by MA and vacuum hot-pressing sintering (VHPS) technique on Q235 steel [11]. CoCrFeMoNi coatings were suggested for diamond tool applications having higher hardness (350–520 HV) than several commonly used metal matrices [12]. Furthermore, the AlCoCrFeNi HEA powder/epoxy composite was found to have excellent microwave shielding effectiveness due to the relatively small powder size and its flake-like shape [8, 13]. Similarly, MA FeCoNiCrAl HEA powders were found to have potential as materials that can absorb electromagnetic waves [14]. Fine-scale precipitation, similar to that seen in superalloys, was also shown in AlCoCuNiZn HEA indicating a desirable high thermal stability [15].
One of the very first attempts to employ MA in the fabrication of HEAs was reported producing nanocrystalline AlCrCuFeTiZn HEA [16]. Phase evolution and its stability are among the fundamental aspects of HEA current research. Shivam et al. have reported a single-phase BCC solid solution of AlCoCrFeNi HEA after 30 h of high energy ball milling. However, the alloy was thermally unstable as it precipitated Ni3Al prototype L12 phase as low as 350 ºC and ordered BCC (B2), L12 and sigma phases were evident after high-temperature annealing [17].
An (Fe40Mn25Cr20Ni15)100−x Alx HEA was produced with varying Al content (x = 0–14 at. %) using vacuum arc melting and casting. A mixture of FCC and BCC/B2 structure was reported at small Al additions (x = 2−6 at.%) while a fully BCC/B2 structure resulted with higher additions (x = 10−14 at.%) [18]. However, the formation of a stable FCC phase was reported in nanocrystalline CoCrFeNi and CoCrFeMnNi HEAs produced by MA followed by spark plasma sintering (SPS) [19].
However, the study of HEAs is still regarded to be in its early stages. Although 3D transition metal HEAs and refractory HEAs have exhibited several promising features and qualities, additional development in their processing and properties is necessary before they can compete with materials like superalloys [8].
The aim of this work is to synthesize Fe34.9Ni30.2Mn18.6Cr9.3Al7 non-equiatomic HEA by MA investigating the effect of milling time: 1, 5, 10, 15 and 25 h. Phase evolution and stability will be studied through XRD and SE and BSE images. In addition, particle size analysis, crystallite size, lattice strain and d-spacing for all specified milling times were calculated. For comparison, a similar Al free alloy was fabricated by MA and milled for 25 h to evaluate the role of Al at optimized milling conditions.
2 Experimental Procedures
High-purity elemental powders of Al (Research Lab, 99.7%), Fe (TPC, 99.7%), Ni (Alpha products, 99.8%), Mn (Prolabo, 99.9%), Cr (Aldrich, 99%), and particles size less than 45 µm were used as the starting materials for the studied alloys. The elemental powders were mechanically milled with non-equiatomic ratio, as shown in Table 1 for the two studied compositions Fe34.9Ni30.2Mn18.6Cr9.3Al7 and Fe37.5Ni32.5Mn20Cr10 (Al-free) referred as Al7 and Al0, respectively, in the following text. Milling was performed in high-energy planetary ball mill (Fritsch Pulverisette 5) using hardened stainless-steel vials (each 250 ml volume) and hardened stainless-steel balls (10 mm dia.). A 30:1 ball to powder weight ratio and 350 rpm rotational speed were used. 1 wt% of stearic acid was added as a process control agent (PCA) to prevent cold welding. All powder mixtures were prepared and sampled in an Mbraun Ar glove box (O2 ≤ 0.1 ppm, H2O ≤ 0.1 ppm).
All powder samples from different milling times were analyzed by Malvern PANalytical, Empyrean 3 X-ray diffractometer (XRD) with Cu Kα radiation, λ = 1.5406 Å, at a scanning rate of 0.02°/sec and a 2θ range of 5–90°. SEM investigations were performed using Thermo Scientific Quattro S equipped with an energy-dispersive X-ray spectrometer (EDS) at an accelerating voltage of 20 kV. BSE images were captured using Quanta FEG-250 (Thermo Fisher scientific) at an accelerating voltage of 20 kV. Particle size analysis was conducted using Malvern Zetasizer Nano ZS by Dynamic Light Scattering (DLS) process for Al7 as milled powders with different milling times dispersed in ethanol 99.98%. The crystallite size was calculated using the Scherrer formula D = (k λ /β cos θ), and the lattice strain was calculated by Williamson-Hall’s method ε = [β − (k λ / D cos θ)]/4tan θ where D is the crystallite size, K is a constant (K = 0.89), λ is the X-ray wavelength, β is full width at half maximum (FWHM) of the diffraction peak, θ is the angle of diffraction, and ε is the lattice strain.
3 Results and Discussion
3.1 Structure Development with Milling Time
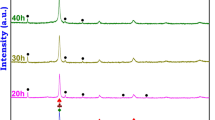
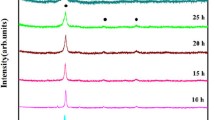
XRD diffractograms of the Al7 alloy sample after different milling times are presented in Fig. 1. The diffraction peaks corresponding to all the alloying elements appear in the sample before milling (0 h). As milling time gradually increased, the diffracted peaks of the pure elements start disappearing and are replaced by new lines which can be indexed to two different phases of FCC and BCC structure solid solutions. The Al peaks disappear first among the other constituent elements followed by a sequential elemental dissolution which could be associated with their relative melting temperatures as well as the accumulated milling energy with time. These factors are known to essentially contribute to the atomic diffusion needed for phase/s formation [20].
Figure 1 reveals that phase formation can start after five hours of milling and the peaks of an FCC phase can be distinguished. However, milling up to 25 h clearly shows the formation of a BCC phase. Increasing the induced strain with milling could be responsible for such transformation which appears to be strain dependent. It is generally possible to point out that the peaks of the FCC phase are shifted to the right towards the Ni lines while the late formed BCC phase is shifted towards Cr lines. Similar behavior has been observed during elements dissolution at different stages of HEAs processed by MA [21].
Increasing the milling time also resulted in peak broadening, indicating a finer grain refinement and more lattice strain. The lattice parameters are estimated to be 0.3598 nm and 0.2920 nm for the FCC and BCC phases, respectively. The obtained FCC lattice constant is comparable to that of nickel element, 0.3529 nm, which can be associated to the small difference in atomic size between Ni, Fe, and Cr. Therefore, the FCC phase is expected to be based on Ni and Fe atoms.
Figure 2 shows the variation of the d200 measured values with milling time based on XRD data. The interplanar distance for the (200) plane, i.e., d200, is measured as a function of milling time to trace phase/s evolution during the milling process. The choice of (200) plane instead of the highest intensity peak (111) is to avoid the overlap between the latter and the BCC (110) plane. The observed sharp increase in d-value in the early milling stage is attributed to the fast progress of the solid solution phase formation reaching saturation between 15 and 25 h milling.
The increase of d-value can be associated with a rapid rise in the calculated lattice strain reaching a maximum value of 0.8% within the milled powder (see Fig. 3). Contrary to lattice strain increase, the crystallite size decreases with milling and reaches a minimum value of 5.7 nm (Fig. 3). From the peaks intensity changes of the two co-existing phases, it is observed that the FCC phase is dominant with a higher volume fraction compared to the BCC phase after 25 h milling which can be attributed to two different rates of phase formation, similar observation has been reported for HEAs processed by MA [22]. On the other hand, the relative lower BCC phase volume fraction can also be associated with the relatively smaller weight percent of Cr, Mn, and Al elements in the alloy composition, in which they are assumed to be the basic elements for forming this phase, as will be indicated later.
Particles size analysis was performed after 1, 5, 10, 15 and 25 h milling time using dynamic light scattering (DLS) method. The results obtained are shown in Table 2 indicating the existence of two main average particles sizes. The small one is in the nano-size range of 200–350 nm while the large one ranges between 1.8 and 3.9 µm depending on the milling time. No possible size distribution values were obtained for each single milling duration for the large size particles formation. However, for small particles, a high intensity distribution over a very small particles size range is observed. The relatively small change with milling times could mean that a possible critical level of microstrain was reached at early milling times so that the particle size remained stable. This may indicate that a steady state with milling time was attained [23]. The small particle size stability with accumulated milling strain up to 25 h can also be an indication of high-strength particles. On the other hand, the large particles show relatively higher size fluctuation during the different milling stages with a final size close to that measured using SEM. Such size fluctuation can be due to its relatively soft character as solid solution particles which undergo the cycles of heavy deformation, cold welding, and fracture during milling duration [24]. The possible fine particles adherence to the larger ones can also, to some extent, affect the degree of the measurement accuracy [25].
3.2 Phase Stability and Processing Route
It is generally suggested that in order to increase the strength of FeCoMnCrNi HEAs, Al-addition would be an effective main factor for the improvement of yield stress [26]. This can occur through the introduction of a BCC second phase where Al is assumed to be essential for its formation. The large size difference between Al atoms and the other constituent elements is expected to produce enough lattice strain to destabilize the existing FCC phase. The original FCC phase then undergoes a partial transformation producing a dual mixture of FCC and BCC phases.
The XRD of Al7 sample compared to the Al0 alloy after 25 h milling is presented in Fig. 4, showing similar patterns. It is observed that the Al0 sample has a dual-phase structure following milling as well. This observation suggests that the BCC phase formation is not necessarily due to Al addition, whereas its role could be replaced by the strain induced by milling in the present HEA composition. Therefore, milling the quaternary mixture (FeNiMnCr) produced not only a single FCC solid solution but also a second BCC phase was observed. The second-phase formation can be associated with Cr in the Al0 alloy. This has been attributed to the positive enthalpy value between both Fe–Cr and Ni–Cr leading to phase separation and consequently Cr-phase precipitation [27].
Another physical parameter has been introduced to predict phase/s formation and stability that is mainly derived from the Hume-Rothery rule [28]. This parameter is related to the atomic size difference (δ) and given by:
where n is the number of constituent elements, \(c_{i}\) and \(r_{i}\) represents the atomic percentage and atomic radius of the ith element, repectively, and \(\overline{r} = \mathop \sum \limits_{i = 1}^{n} c_{i} r_{i}\) is the average atomic radius. The estimated values for the present alloy changes from 1.08 to 3.62% when Al is added to the quaternary alloy predicting the solid solution formation [29]. Al-atom having the largest radius compared to the transition elements of the alloy indicating possible lattice distortion providing a strain energy which can lead to the FCC to BCC transformation. Although the δ -criterion can reasonably predict the formation of solid solution, it appears ineffective in determining its exact crystal structure type [26].
3.3 Phase Stability Prediction
The expected phase/s formation and their stability were predicted using JMatPro 13.1 software, this program uses thermodynamic and thermo-kinetic data model to calculate the phase equilibria, solidification behavior of alloys [30] assuming an equilibrium cooling during solidification. The calculated phase/s weight percentage as a function of temperature are shown for the Al0 and Al7 systems (Fig. 5a, and b), respectively.
The Al0 alloy indicated an FCC-austenitic phase at the beginning of solidification which started decomposing around 400 °C forming a ferritic BCC phase with 35% volume fraction. With Al addition, the solidification sequence did not produce similar phases to the Al-free alloy (Fig. 5b). The austenitic phase started decomposing at a higher temperature of about 600 °C, i.e., Al destabilized the FCC phase to the benefit of both the ferrite and alpha phases with a relatively higher volume fraction for the ferrite. Despite the different processing route, the predicted phase/s are not much far from those observed with MA route.
3.4 Powder Micromorphology
SEM images for the Al7 alloy milled powder are shown in Fig. 6 after 25 h of milling. Two different types of morphology with relatively different sized particles can be observed. The smaller discrete particles with irregular shape and average size of 300–500 nm appear in BSE images (Fig. 6c and d), as lighter areas compared with the relatively larger gray particles of about 3–5 um. The large particles have a plate-like shape with smooth surfaces and some slip lines are observed. The latter are likely to result from the accumulated plastic deformation during milling with repetitive collisions with the grinding balls. However, the smaller particles appear to result from the fragmentation of welded particles during milling. The different size and morphology of powder particles can be an indication of their different compositions and in turn their differing mechanical behavior during MA processing. The bimodal morphology is evident after only one hour of milling which strongly suggests an incomplete phase formation (see Table 3) by EDS analysis. The EDS analysis after higher milling times showed very small fluctuations in elements concentrations despite their size difference, as shown in Table 3 and Fig. 7. However, a rather homogeneous phase distribution for the milled particles is essential for any subsequent sintering processes. The effect of milling time can be observed by comparing Fig. 6c and d with Fig. 7c and d where the agglomerated particles in the early hours of milling were fractured by milling.
Phase/s evolution during MA and consolidation are generally different compared to that developed by melting and casting for similar alloy compositions. The early weakening of Al-diffraction peaks in the present work is due to the rapid dissolution of this element during compound formation and also suggests a sequential nature for dissolving the constitution elements during phase formation. Higher melting point elements such as Cr, Fe, and Ni with their expected lower diffusivity and dissolution were dissolved at late milling stages.
The mixing enthalpy between component elements is generally known to affect the solubility in HEAs solid solution, for example, a positive enthalpy ΔH will have the tendency to segregate rather than forming compound [31]. On the other hand, it has been indicated that the mixing enthalpies between component elements will not have direct evidence for component dissolution during milling, e.g., Ni–Al with a rather low ΔH value of − 22 kJ/mol will not form a compound during powder processing [32]. This is also reported by Chen et al. that confirm that competition between the enthalpy and mixing configuration entropy can be a determining factor for phase formation [33]. Then, alloys with high entropy do not necessarily result in a high-entropy alloy and an intermetallic component can be formed [34]. It is equally important to indicate that the element weight content can also influence the rate of dissolution [19], and it is shown that for a single-phase formation from Fe and Ni, a 20 h milling is needed. However, when Co is added to Fe and Ni or CoCrNiFe system only 15 h is needed. These results can also suggest that other factors might be significant to the rate of dissolution, in addition to factors such as the mixing entropy.
Valence electron concentration (VEC) criteria are often used to predict phase/s evolution with HEAs composition change and can be estimated using the formula:
where \(c_{i}\) and \(\left( {{\text{VEC}}} \right)_{i}\) represent the atomic percentage and valence electron concentration of the ith component, respectively [35]. It is generally indicated that when VEC < 6.87, a BCC-type structure is stable while increasing the value to > 8, an FCC structure will take place while a mixture of both BCC and FCC structures is suggested for the range in between those two values [35, 36]. A calculated value of 7.88 VEC for the present system agrees well with that prediction where a two-phase mixture microstructure is obtained. The increasing addition of Al (VEC = 3) and Cr (VEC = 6) is expected to reduce the VEC of the alloy and subsequently enhance the two-phase mixture transformation tendency to single phase in accordance with the VEC concept. Similar results in AlxCoCrMnFe HEAs suggested that the formed solid solution changed from FCC and BCC mixture to a single phase with BCC structure when increasing the Al equivalent × from 0.5 to 1.5 at.% [37]. VEC criterion is relatively accurate in production phase formation tendency. However, it should be noted that during MA process, collision energy, milling time, cold-welding and fracture affect the dissolution process, which influence the solid solution content and phase stability [38]. Therefore, phase formation and stability in HEAs under milling processing conditions require more factors other than the atomic bonding [26]. The induced high strain during MA appears to be effective in enhancing the highly observed heterogeneity on both elements and phases distribution taking place in the powder microstructure.
4 Conclusions
-
Fe34.9Ni30.2Mn18.6Cr9.3Al7 and Fe37.5Ni32.5Mn20Cr10 HEAs were successfully synthesized using mechanical alloying technique and showed to have two distinct solid solution phases of FCC and BCC structures after 25 h milling.
-
A similar trend of gradual increase is noticed in both d-spacing values and the accumulated lattice strain in the milled powders along with increasing milling time.
-
A strain-induced transformation is evident after 25 h milling leading to BCC phase formation and a dual-phase HEA structure in both the Al-bearing and Al-free variations of the studied HEA.
-
The BCC phase formation in the present HEA is therefore found to be strain induced and not necessarily due to Al addition and was associated with a decrease in the accumulated strain (ca.32%) confirming a strain-induced transformation in the present HEA.
-
An estimated value of 7.88 VEC for the present system agrees with VEC prediction criteria and a two-phases mixture microstructure is obtained after 25 h milling.
-
The acquired structure homogeneity of the obtained microconstituents and phase/s distribution can be a merit of mechanical alloying over casting route.
-
Mechanical alloying for 25 h decreased the crystallite size of Fe34.9Ni30.2Mn18.6Cr9.3Al7 alloy down to 5.7 nm, and particle size was also refined to about 300–500 nm for BCC structure and 3–5 μm for FCC structure.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cantor B, Chang I T H, Knight P, and Vincent A J B, Mater Sci Eng A 375–377 (2004) 213. https://doi.org/10.1016/j.msea.2003.10.257
Yeh J W, Chen S K, Lin S J, Gan J Y, Chin T S, Shun T T, Tsau C H, and Chang S Y, Adv Eng Mater 6 (2004) 299. https://doi.org/10.1002/adem.200300567
Murty B S, Yeh J W, and Ranganathan S, in High-Entropy Alloys, Butterworth-Heinemann, Oxford (2014), p 13–35. https://doi.org/10.1016/B978-0-12-800251-3.00002-X
Suryanarayana C and Ivanov E, Mater Sci Technol 2003 Meet (2003) 169. https://doi.org/10.1002/9781118984239.ch17
Kumar A, Singh A, and Suhane A, J Mater Res Technol 17 (2022) 2431. https://doi.org/10.1016/j.jmrt.2022.01.141
Suñol J J, Metals (Basel) 11 (2021) 10. https://doi.org/10.3390/books978-3-0365-2118-3
Hrapkowicz A B, Lesz S, Kremzer M, Pakieła W, Bartłomiej H, Sabina L, Marek K, Hrapkowicz B, Lesz S, Kremzer M, Karolus M, and Pakieła W, Bull Pol Acad Sci (2021). https://doi.org/10.24425/bpasts.2021.137587
Vaidya M, Muralikrishna G M, and Murty B S, J Mater Res 34 (2019) 664. https://doi.org/10.1557/jmr.2019.37
Sharma A, Coatings (2021). https://doi.org/10.3390/coatings11040372
Tian L, Fu M, and Xiong W, Materials (Basel) (2018). https://doi.org/10.3390/ma11020320
Shang C, Axinte E, Ge W, Zhang Z, and Wang Y, Surf Interfaces 9 (2017) 36. https://doi.org/10.1016/j.surfin.2017.06.012
Zhang M, Zhang W, Liu Y, Liu B, and Wang J, Powder Metall 61 (2018) 123. https://doi.org/10.1080/00325899.2018.1429044
Zhang Y, Zhang B, Li K, Zhao G L, and Guo S M, J Alloys Compd 734 (2018) 220. https://doi.org/10.1016/j.jallcom.2017.11.044
Yang P, Liu Y, Zhao X, Cheng J, and Li H, Adv Powder Technol 27 (2016) 1128. https://doi.org/10.1016/j.apt.2016.03.023
Mohanty S, Gurao N P, and Biswas K, Mater Sci Eng A 617 (2014) 211. https://doi.org/10.1016/j.msea.2014.08.046
Varalakshmi S, Kamaraj M, and Murty B S, J Alloys Compd 460 (2008) 253. https://doi.org/10.1016/j.jallcom.2007.05.104
Shivam V, Basu J, Shadangi Y, Singh M K, and Mukhopadhyay N K, J. Alloys Compd 757 (2018) 87. https://doi.org/10.1016/j.jallcom.2018.05.057
Stepanov N D, Shaysultanov D G, Chernichenko R S, Tikhonovsky M A, and Zherebtsov S V, J Alloys Compd 770 (2019) 194. https://doi.org/10.1016/j.jallcom.2018.08.093
Vaidya M, Karati A, Marshal A, Pradeep K G, and Murty B S, J Alloys Compd 770 (2019) 1004. https://doi.org/10.1016/j.jallcom.2018.08.200
Chen Q, Zhang H, Zhou S, Cai Y, Li X, and Xu Y, J Alloys Compd 802 (2019) 493. https://doi.org/10.1016/j.jallcom.2019.06.247
Bahrami A, Mohammadnejad A, and Sajadi M, J Alloys Compd 862 (2021) 158577. https://doi.org/10.1016/j.jallcom.2020.158577
Chen W, Fu Z, Fang S, Xiao H, and Zhu D, Mater Des 51 (2013) 854. https://doi.org/10.1016/j.matdes.2013.04.061
Zhao Y H, Lu K, and Zhang K, Phys Rev B-Condens Matter Mater Phys 66 (2002) 1. https://doi.org/10.1103/PhysRevB.66.085404
Aghaali V, Ebadzadeh T, Karimi Z, Kazemzadeh A, and Marzbanrad E, J Mater Res Technol 12 (2021) 749. https://doi.org/10.1016/j.jmrt.2021.02.089
Lü L, and Lai M O, Mechanical Alloying, Springer, New York, NY (1998). https://doi.org/10.1007/978-1-4615-5509-4
He J Y, Liu W H, Wang H, Wu Y, Liu X J, Nieh T G, and Lu Z P, Acta Mater 62 (1), (2014) 105–113. https://doi.org/10.1016/j.actamat.2013.09.037
Portnoi V K, Leonov A V, Filippova S E, Kuznetsov V N, Streletskii A N, Logacheva A I, and Gusakov M S, Inorg Mater 52 (2016) 529. https://doi.org/10.1134/S0020168516050125
Zhang Y, Zhou Y J, Lin J P, Chen G L, and Liaw P K, Adv Eng Mater 10 (2008) 534. https://doi.org/10.1002/adem.200700240
Zhang Y, Chen G L, and Gan C L, J ASTM Int 7 (5), (2010) 1–8. https://doi.org/10.1520/JAI102527
Saunders N, Schillé J, Guo Z, and Lasne P, Procedia Manuf 15 (2018) 372. https://doi.org/10.1016/j.promfg.2018.07.232
Wu P H, Liu N, Zhou P J, Peng Z, Du W D, Wang X J, and Pan Y, Mater Sci Technol 32 (2016) 576. https://doi.org/10.1179/1743284715Y.0000000127
Huang M, Jiang J, Wang Y, Liu Y, and Zhang Y, Mater Des 217 (2022) 110637. https://doi.org/10.1016/j.matdes.2022.110637
Chen Y L, Hu Y H, Hsieh C A, Yeh J W, and Chen S K, J Alloys Compd 481 (2009) 768. https://doi.org/10.1016/j.jallcom.2009.03.087
Campo K N, de Freitas C C, da Fonseca E B, and Caram R, Mater Charact (2021). https://doi.org/10.1016/j.matchar.2021.111260
Guo S, Ng C, Lu J, and Liu C T, J Appl Phys (2011). https://doi.org/10.1063/1.3587228
Cheng H, Liu X, Tang Q, Wang W, Yan X, and Dai P, J Alloys Compd 775 (2019) 742. https://doi.org/10.1016/j.jallcom.2018.10.168
Ruiz-Esparza-Rodríguez M A, Garay-Reyes C G, Estrada-Guel I, Hernández-Rivera J L, Cruz-Rivera J J, Gutiérrez-Castañeda E, Gómez-Esparza C D, and Martínez-Sánchez R, J Alloys Compd 882 (2021) 160770. https://doi.org/10.1016/j.jallcom.2021.160770
Suryanarayana C, Prog Mater Sci 46 (2001) 1–184. https://doi.org/10.1016/S0079-6425(99)00010-9
Acknowledgements
The authors are grateful to the Science and Technology Development Fund (STDF) funding project No. 22854, which made facilities available at the Sustainable Energy Lab, Suez University.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abbas, M.A., Sadek, W.M. & Ibrahim, S.A. Effect of Milling Time on the Structure Stability of FeMnNiCrAl Non-equiatomic High-Entropy Alloy. Trans Indian Inst Met (2024). https://doi.org/10.1007/s12666-024-03274-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12666-024-03274-3