Abstract
Clathrate hydrates (or gas hydrates) belong to the class of crystalline guest–host compounds where water molecules act as hosts forming cage-like structures to entrap small guest molecules with suitable size, such as methane, ethane, CO2, and N2. The design of oil and gas production facilities and hydrate-based applications (desalination, energy storage/transportation, etc.) require a clear understanding of the thermodynamics and kinetics of gas hydrate formation. The current work encapsulates the fundamentals of thermodynamics, nucleation, and growth kinetics of gas hydrates critical in developing processes for gas hydrate-based applications. In the thermodynamics part, the original van der Waals and Platteeuw model and its modifications are discussed, and the challenges with inhibited hydrate phase equilibria measurements and predictions are highlighted. Nucleation and growth kinetics of gas hydrates are also briefly reviewed to present the current state of understanding in this field. While gas hydrate thermodynamics is reasonably well understood, the understanding of nucleation and growth kinetics of gas hydrates is relatively poor. However, molecular simulations have advanced our understanding considerably in recent years.

Adapted from Warrier et al. [3]





Modified from Ward [37]

Reproduced with permission from Ward [37]




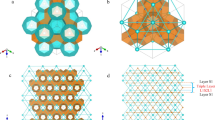
Adapted from Khan et al. [10] (color Figure Online)
Similar content being viewed by others
References
Sloan E D, and Koh C A, Clathrate Hydrates of Natural Gases, 3rd edn, CRC Press Boca Raton FL (2007), Hardcover ISBN: 9780849390784. https://doi.org/10.1201/9781420008494
Koh C A, Sloan E D, Sum A K, and Wu D T, Annu Rev Chem Biomol Eng 2 (2011) 237. https://doi.org/10.1146/annurev-chembioeng-061010-114152
Warrier P, Khan M N, Srivastava V, Maupin C M, and Koh C A, J Chem Phys 145 (2016) 211705. https://doi.org/10.1063/1.4968590
Davy VIII H, Philos Trans R Soc Lond 101 (1811) 155. https://doi.org/10.1098/rstl.1811.0008
Hammerschmidt E, Ind Eng Chem 26 (1934) 851. https://doi.org/10.1021/ie50296a010
Sloan E D, Molecules 26 (2021) 4476. https://doi.org/10.3390/molecules26154476
Chatti I, Delahaye A, Fournaison L, and Petitet J P, Energy Convers Manag 46 (2005) 1333. https://doi.org/10.1016/j.enconman.2004.06.032
Ripmeester J A, and Alavi S, Clathrate Hydrates: Molecular Science and Characterization, Volume 1, Wiley-VCH GmbH (2022), E-Book ISBN: 9783527695058. https://doi.org/10.1002/9783527695058
Max M D, and Pellenbarg R E, Desalination through gas hydrate, U.S. Patent US6158239A (2000). https://patents.google.com/patent/US6158239A/en. Accessed 4 Nov 2022
Khan M N, Peters C J, and Koh C A, Desalination 468 (2019) 114049. https://doi.org/10.1016/j.desal.2019.06.015
Linga P, Kumar R, Lee J D, Ripmeester J, and Englezos P, Int J Greenh Gas Control 4 (2010) 630. https://doi.org/10.1016/j.ijggc.2009.12.014
Gudmundsson J, Andersson V, Levik O, and Mork M, Ann NY Acad Sci 912 (2000) 403. https://doi.org/10.1111/j.1749-6632.2000.tb06794.x
Babu P, Linga P, Kumar R, and Englezos P, Energy 85 (2015) 261. https://doi.org/10.1016/j.energy.2015.03.103
Thomas S, and Dawe R A, Energy 28 (2003) 1461. https://doi.org/10.1016/S0360-5442(03)00124-5
Takaoki T, Iwasaki T, Katoh Y, Arai T, and Horiguchi K, Use of hydrate pellets for transportation of natural gas I - Advantage of pellet form of natural gas hydrate in sea transportation, Proc. 4th Int. Conf. Gas Hydrates, Yokohama (2002) 5. https://cir.nii.ac.jp/crid/1571417125434354944. Accessed 4 Nov 2022
Linga P, Kumar R, and Englezos P, J Hazard Mater 149 (2007) 625. https://doi.org/10.1016/j.jhazmat.2007.06.086
Warrier P, Khan M N, Carreon M A, Peters C J, and Koh C A, J Renew Sustain Energy 10 (2018) 034701. https://doi.org/10.1063/1.5019967
Khan M N, Warrier P, Peters C J, and Koh C A, Energies 15 (2022) 966. https://doi.org/10.3390/en15030966
Strobel T A, Taylor C J, Hester K C, Dec S F, Koh C A, Miller K T, and Sloan E D, J Phys Chem B 110 (2006) 17121. https://doi.org/10.1021/jp062139n
Zhang Y, Bhattacharjee G, Zheng J, and Linga P, Chem Eng J 427 (2022) 131771. https://doi.org/10.1016/j.cej.2021.131771
Boswell R, and Collett T S, Energy Environ Sci 4 (2011) 1206. https://doi.org/10.1039/C0EE00203H
Waite W F, Ruppel C D, Boze L-G, Lorenson T D, Buczkowski B J, McMullen KY, and Kvenvolden K A, Preliminary global database of known and inferred gas hydrate locations: U.S. Geological Survey data release, (2020). https://doi.org/10.5066/P9llFVJM
Collett T S, Boswell R, Waite W F, Kumar P, Roy S K, Chopra K, Singh S K, Yamada Y, Tenma N, Pohlman J, Zyrianova M, NGHP Expedition 02 Scientific Party, Mar Pet Geol 108 (2019) 39. https://doi.org/10.1016/j.marpetgeo.2019.05.023
Khan M N, Warrier P, Peters C J, and Koh C A, Fluid Phase Equilib 463 (2018) 48. https://doi.org/10.1016/j.fluid.2018.01.014
Ke W, and Kelland M A, Energy Fuels 30 (2016) 10015. https://doi.org/10.1021/acs.energyfuels.6b02739
Yu C, Yue C, Sun B, Yang X, Ji J, Meng Z, and Chen L, Energy Fuels 36 (2022) 10685. https://doi.org/10.1021/acs.energyfuels.2c01285
Ripmeester J A, and Alavi A, Curr Opin Solid State Mat Sci 20 (2016) 344. https://doi.org/10.1016/j.cossms.2016.03.005
Yin Z, Khurana M, Tan H K, and Linga P, Chem Eng J 342 (2018) 9. https://doi.org/10.1016/j.cej.2018.01.120
Salmin D C, Estanga D, and Koh C A, Fuel 319 (2022) 122862. https://doi.org/10.1016/j.fuel.2021.122862
Sayani J K S, Lal B, and Pedapati S R, Arch Comput Methods Eng 29 (2022) 2171. https://doi.org/10.1007/s11831-021-09651-1
Zerpa LE, A Practical Model to Predict Gas Hydrate Formation, Dissociation and Transportability in Oil and Gas Flowlines, PhD Thesis, Colorado School of Mines (Golden, Colorado USA) 2013. https://repository.mines.edu/handle/11124/78776. Accessed 2 Nov 2022.
Clathrate Hydrate Physical Property Database, https://gashydrates.nist.gov/. Accessed 2 Nov 2022.
Khan MNK, Phase equilibria modeling of inhibited gas hydrate systems including salts: applications in flow assurance, seawater desalination and gas separation, PhD Thesis, Colorado School of Mines (Golden, Colorado, USA) 2016, https://repository.mines.edu/handle/11124/170013. Accessed 2 Nov 2022.
Hu Y, Makogon T Y, Karanjkar P, Lee K-H, Lee B R, and Sum A K, J Chem Eng Data 62 (2017) 1910. https://doi.org/10.1021/acs.jced.7b00292
Khan M N, Warrier P, Peters C J, and Koh C A, J Nat Gas Sci Eng 35 (2016) 1355. https://doi.org/10.1016/j.jngse.2016.03.092
Cai J, Wang X-H, Xiao P, Tang H, Liu B, Sun C-Y, and Chen G-J, Fuel 333 (2023) 126282. https://doi.org/10.1016/j.fuel.2022.126282
Ward Z T, Phase Equilibria of gas hydrates containing hydrogen sulfide and carbon dioxide, PhD Thesis, Colorado School of Mines (Golden, Colorado, USA) 2015. https://repository.mines.edu/handle/11124/20280. Accessed 28 Jun 2023.
Lafond P G, Olcott K A, Sloan E D, Koh C A, and Sum A K, J Chem Thermodyn 48 (2012) 1. https://doi.org/10.1016/j.jct.2011.12.023
Nielsen R B, and Bucklin R W, Hydrocarbon Process., 62 (1983) 71–78, https://www.osti.gov/biblio/5874635
Carroll J, Natural Gas Hydrates: A Guide for Engineers, 4th Edition, Gulf Professional Publishing, 2020. ISBN: 9780128217719. https://doi.org/10.1016/C2019-0-04277-X
van der Waals J H, and Platteeuw J C, in Prigogine I (ed), Advances in Chemical Physics, Volume 2, Wiley. 1958, ISBN:9780470143483. https://doi.org/10.1002/9780470143483.ch1
Ballard A L, and Sloan E D, Fluid Phase Equilib 194–197 (2002) 371. https://doi.org/10.1016/S0378-3812(01)00697-5
Parrish W R, and Prausnitz J M, Ind Eng Chem Process Des Dev 11 (1972) 26. https://doi.org/10.1021/i260041a006
Klauda J B, and Sandler S I, Chem Eng Sci 58 (2003) 27. https://doi.org/10.1016/S0009-2509(02)00435-9
Martin A, and Peters C J, J Phys Chem B 113 (2009) 7548. https://doi.org/10.1021/jp807367j
Poling B E, Prausnitz J M, and O’Connell J P, The Properties of Gases and Liquids, 5th Edition, McGraw-Hill Education, New York (2001). ISBN: 9780070116825. DOI: https://doi.org/10.1036/0070116822
Debye P, and Hückel E, Physikalische Z 24 (1923) 185.
Bromley L A, AIChE J 19 (1973) 313. https://doi.org/10.1002/aic.690190216
Pitzer K S, J Phys Chem 77 (1973) 268. https://doi.org/10.1021/j100621a026
Khan M N, Warrier P, Creek J L, Peters C J, and Koh C A, J Nat Gas Sci Eng 94 (2021) 104083. https://doi.org/10.1016/j.jngse.2021.104083
Meragawi S E, Diamantonis N I, Tsimpanogiannis I N, and Economou I G, Fluid Phase Equilib 413 (2016) 209. https://doi.org/10.1016/j.fluid.2015.12.003
Lira C T, Elliott R, Gupta S, and Chapman W G, Ind Eng Chem Res 61 (2022) 15678. https://doi.org/10.1021/acs.iecr.2c02058
Russo J, Romano F, and Tanaka H, Nat Mat 13 (2014) 733. https://doi.org/10.1038/nmat3977
Cox S J, Kathmann S M, Slater B, and Michaelides A, J Chem Phys 142 (2015) 184704. https://doi.org/10.1063/1.4919714
Ohmura R, Ogawa M, Yasuoka K, and Mori Y H, J Phys Chem B 107 (2003) 5289. https://doi.org/10.1021/jp027094e
Takeya S, Hori A, Hondoh T, and Uchida T, J Phys Chem B 104 (2000) 4164. https://doi.org/10.1021/jp993759+
Guo G-J, and Rodger P M, J Phys Chem B 117 (2013) 6498. https://doi.org/10.1021/jp3117215
Bagherzadeh S A, Alavi S, Ripmeester J, and Englezos P, J Chem Phys 142 (2015) 214701. https://doi.org/10.1063/1.4920971
Maeda N, J Phys Chem C 122 (2018) 11399. https://doi.org/10.1021/acs.jpcc.8b02416
Zeng H, Wilson L D, Walker L K, and Ripmeester J A, J Am Chem Soc 128 (2006) 2844. https://doi.org/10.1021/ja0548182
Zeng H, Moudrakovski I L, Ripmeester J A, and Walker V K, AIChE J 52 (2006) 3304. https://doi.org/10.1002/aic.10929
Sloan E D, and Fleyfel F, AIChE J 37 (1991) 1281. https://doi.org/10.1002/aic.690370902
Radhakrishnan R, and Trout B L, J Chem Phys 117 (2002) 1786. https://doi.org/10.1063/1.1485962
Jacobson L C, Hujo W, and Molinero V, J Phys Chem B 114 (2010) 13796. https://doi.org/10.1021/jp107269q
Jacobson L C, Hujo W, and Molinero V, J Am Chem Soc 132 (1811) 11806. https://doi.org/10.1021/ja1051445
Walsh M R, Koh C A, Sloan E D, Sum A K, and Wu D T, Science 326 (2009) 1095. https://doi.org/10.1126/science.1174010
Zhang Z, Kusalik P G, and Guo G-J, Phys Chem Chem Phys 20 (2018) 24535. https://doi.org/10.1039/C8CP04466J
Guo G-J, and Zhang Z, Commun Chem 4 (2021) 102. https://doi.org/10.1038/s42004-021-00539-6
Zhang Z, Walsh M R, and Guo G-J, Phys Chem Chem Phys 17 (2015) 8870. https://doi.org/10.1039/C5CP00098J
Bai D, Chen G, Zhang X, and Wang W, Langmuir 27 (2011) 5961. https://doi.org/10.1021/la105088b
Bai B, Chen G, Zhang X, Sum A K, and Wang W, Sci Rep 5 (2015) 12747. https://doi.org/10.1038/srep12747
Englezos P, Kalogerakis N, Dholabhai P D, and Bishnoi P R, Chem Eng Sci 42 (1987) 2647. https://doi.org/10.1016/0009-2509(87)87015-X
Englezos P, Kalogerakis N, Dholabhai P D, and Bishnoi P R, Chem Eng Sci 42 (1987) 2659. https://doi.org/10.1016/0009-2509(87)87016-1
Freer E M, Selim M S, and Sloan E D, Fluid Phase Equilib 185 (2001) 65. https://doi.org/10.1016/S0378-3812(01)00457-5
Mochizuki T, and Mori Y H, J Cryst Growth 290 (2006) 642. https://doi.org/10.1016/j.jcrysgro.2006.01.036
Vysniauskas A, and Bishnoi P R, Chem Eng Sci 38 (1983) 1061. https://doi.org/10.1016/0009-2509(83)80027-X
Vysniauskas A, and Bishnoi P R, Chem Eng Sci 40 (1985) 299. https://doi.org/10.1016/0009-2509(85)80070-1
Zerpa L E, Sloan E D, Sum A K, and Koh C A, J Pet Sci Eng 98–99 (2012) 122. https://doi.org/10.1016/j.petrol.2012.08.017
Li M, Li K, Yang L, Su Y, Zhao J, and Song Y, J Phys Chem Lett 13 (2022) 400. https://doi.org/10.1021/acs.jpclett.1c03857
Cruz F J A L, and Mota J P B, Phys Chem Chem Phys 23 (2021) 16033. https://doi.org/10.1039/D1CP00893E
Michalis V K, Costandy J, Tsimpanogiannis I N, Stubos A K, and Economou I G, J Chem Phys 142 (2015) 044501. https://doi.org/10.1063/1.4905572
Luis D P, García-González A, and Saint-Martin H, Int J Mol Sci 17 (2016) 378. https://doi.org/10.3390/ijms17060378
Barwood M T J, Metaxas P J, Lim V W S, Sampson C C, Johns M L, Aman Z M, and May E F, Chem Eng J 450 (2022) 137895. https://doi.org/10.1016/j.cej.2022.137895
Zhang Z, Kusalik P G, Wu N, Liu C, and Ning F, ACS Sustain Chem Eng 10 (2022) 11597. https://doi.org/10.1021/acssuschemeng.2c03428
Wang D, Li D, Kelland M A, Cai H, Wang J, Xu P, Lu P, and Dong J, Langmuir 38 (2022) 4774. https://doi.org/10.1021/acs.langmuir.2c00472
Lim V W S, Metaxas P J, Johns M L, Haandrikman G, Crosby D, Aman Z M, and May E F, Chem Eng J 411 (2021) 128478. https://doi.org/10.1016/j.cej.2021.128478
Hall S W, Leines G D, Sarupria S, and Rogal J, J Chem Phys 156 (2022) 2009. https://doi.org/10.1063/5.0080053
Acknowledgements
CAK would like to thank current and past CSM Hydrate Consortium members for their support. PW would like to thank Prof. P C Kapur for his support and guidance during PW’s tenure at the Tata R&D and Design Center, Pune, India (2005-07).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, M.N., Warrier, P. & Koh, C.A. An Overview of Thermodynamics and Growth Kinetics of Gas Hydrate Systems. Trans Indian Inst Met (2023). https://doi.org/10.1007/s12666-023-03095-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12666-023-03095-w