Abstract
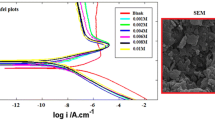
The corrosion inhibition of magnesium by N,N-bis (salicylidene)-2-hydroxy-1, 3-propanediamine Schiff base has been studied in 0.01 M HCl using potentiodynamic polarization and electrochemical noise methods. The polarization curves reveals that the Schiff base used in this study is mixed-type corrosion inhibitor for magnesium and the inhibition efficiency increases as the inhibitor concentration increases. The results obtained by analysis of the electrochemical noise data in frequency domain and those obtained by potentiodynamic polarization measurements are in good agreement. It is found that the Schiff base compound acts by adsorption on the surface and its adsorption follows Fruendlich isotherm. Density function theory calculations shows that stable Mg2(ligand)4+ complex can be formed on the surface due to interaction between the Schiff base ligand and Mg2+ ions. Scanning electron microscope images reveals that the damage of magnesium surface diminishes in the presence of the inhibitor.









Similar content being viewed by others
References
Zhang S, Li Q, Yang X, Zhong X, Dai Y, and Luo F, Mater Character 61 (2010) 269.
Ambat R, and Zhou W, Surf Coat Technol 179 (2004) 124.
El Mahallawy N, Bakkar A, Shoeib M, Palkowski H, and Neubert V, Surf Coat Technol 202 (2008) 5151.
Zhao H, Huang Z, and Cui J, Surf Coat Technol 202 (2007) 133.
Karavaia O V, Bastosa A C, Zheludkevicha M L, Tarybab M G, Lamakab S V, and Fererira M G S, Electrochim Acta 55 (2010) 5401.
Gao H, Li Q, Dai Y, Luo F, and Zhang H X, Corros Sci 52 (2010) 1603.
Gao H, Li Q, Chen F N, Dai Y, Luo F, and Li L Q, Corros Sci 53 (2011) 1401.
Huang D, Hu J, Song G L, and Guo X, Electrochim Acta 56 (2011) 10166.
Issaadi S, Douadi T, Zouaoui A, Chafaa S, Khan M A, and Bouet G, Corros Sci 53 (2011) 1484.
Elsentriecy H H, Azumi K, and Konno H, Surf Coat Technol 202 (2007) 532.
Su H Y, Li W J, and Lin C S, J Electrochem Soc 159 (2012) 219.
Huang Y H, Lee Y L, and Lin C S, J Electrochem Soc 158 (2011) 310.
Seifzadeh D, Basharnavaz H, and Bezaatpour A, Mater Chem Phys 138 (2013) 794.
Thirugnanaselvi S, Kuttirani S, and Emelda A R, Trans Nonferrous Met Soc China 24 (2014) 1969.
Shekaari H, Bezaatpour A, and Elhami R, J Solut Chem 41 (2012) 516.
Granovsky A A, Firefly version 7.1.G. http://classic.chem.msu.su/gran/firefly/index.html
Schmidt M W, Baldridge K K, Boatz J A, Elbert S T, Gordon M S, Jensen J H, Koseki S, Matsunaga N, Nguyen K A, Su S, Windus T L, Dupuis M, and Montgomery J A, J Comput Chem 14 (1993) 1347.
Becke A D, J Chem Phys 98 (1993) 5648.
Schaefer A, Horn H, and Ahlrichs R, J Chem Phys 97 (1992) 2571.
Schaefer A, Huber C, and Ahlrichs R, J Chem Phys 100 (1994) 5829.
Ashassi-Sorkhabi H, Shaabani B, and Seifzadeh D, Appl Surf Sci 239 (2005) 154.
Solmaz R, Corros Sci 52 (2010) 3321.
Ashassi-Sorkhabi H, and Seifzadeh D, J Appl Electrochem 38 (2008) 1545.
Caridade C G, Isabel M, Pereira S, and Brett C M A, Electrochim Acta 49 (2004) 785.
Girija S, Mudali U K, Khatak H S, and Raj B, Corros Sci 49 (2007) 4051.
Lee C C, and Mansfeld F, Corros Sci 40 (1998) 959.
Hegazy M A, Corros Sci 51 (2009) 2610.
Megahed H E, Port Electrochim Acta 29 (2011) 287.
Lagrrenee M, Mernari B, Bouanis M, Traisnel M, and Bentiss F, Corros Sci 44 (2002) 573.
Canul M A P, and Perez P B, Surf Coat Technol 184 (2004) 133.
McQuarrie D A, Statistical Mechanics, Harper Collins Publishers, New York (1976).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seifzadeh, D., Bezaatpour, A., Shamkhali, A.N. et al. Experimental and Theoretical Studies to Examine the Inhibition Effect of a Schiff Base Against Magnesium Corrosion. Trans Indian Inst Met 69, 1545–1555 (2016). https://doi.org/10.1007/s12666-015-0728-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-015-0728-0